Serotransferrin/TF:血液中的铁转运蛋白,新型纳米药物研究潜力靶点!
日期:2024-02-07 11:57:59
2024年1月,Scholar数据库收录了一篇名为“Remyelinating effect driven by transferrin-loaded extracellular vesicles”的研究论文 [1]。该研究旨在评估利用细胞外囊泡(EVs)作为转铁蛋白(Transferrin,Tf)的载体,通过鼻腔途径将Tf输送到中枢神经系统(CNS)的可行性。结果显示,EV Tf通过胞吞作用的途径,进入神经胶质细胞(OPCs),释放出Tf,从而促进OPCs的成熟,进一步揭示了EVs作为TF的功能性纳米载体,能够诱导髓鞘再生。近年来,Serotransferrin/TF修饰的纳米新型药物正成为研究癌症治疗的热门方向之一,它将药物通过载体方式(如外泌体)纳入到递送系统中,靶向癌细胞中高表达的生物靶点。今天,让我们一起了解下这个体液主要的转铁蛋白Tf,以及它在疾病研究中的应用潜力!
1. 什么是血清转铁蛋白(Serotransferrin,Tf)?
1.1 血清转铁蛋白的结构
血清转铁蛋白(Serotransferrin,又称转铁蛋白Transferrin或TRF、Tf)是血浆中的一种β球蛋白,也是血液中主要的铁转运蛋白。血清转铁蛋白最早由Holmberg和Laurell两位研究者发现的,由679个氨基酸残基构成,分子量约为79kD,由两个具有高度同源性结构的N端结构域(336个氨基酸残基)和C端结构域(343个氨基酸残基)构成。每个Tf能结合两个金属离子,可与金属离子Fe3+、Cu2+、Co3+、Cr3+等结合,其中与Fe3+结合能力强,但不能与Fe2+结合。Fe3+分别与结构域中2个酪氨酸、1个天冬氨酸和1个组氨酸结合,形成一个稳定的八面体结构(图1) [2-4]。
1.2 血清转铁蛋白的表达和功能
TF是一种特异性结合并转运铁的糖蛋白,广泛存在于多种组织和器官中。血液循环中的转铁蛋白主要由肝脏细胞合成,同时支持细胞、室管膜、少突神经胶质细胞等组织细胞也能合成少量的转铁蛋白。机体内除了血清含有转铁蛋白外,其他体液中也包含一定量的转铁蛋白,如血浆、脑脊液、胆汁、淋巴液、羊水和乳汁。在没有受到其它因素刺激的情况下,血清中的转铁蛋白浓度非常稳定 [5-7]。
Tf提供了机体中绝大部分的铁,其主要生理功能就是把铁输送到成红血细胞,以用于血红蛋白的合成,或者将三价铁离子从吸收和储蓄的地方运输到其他需铁部位。然而,铁离子的缺乏或过载同样会引起机体组织器官的损伤。因此,维持铁离子平衡是维持生命活动的关键之一。在这个过程中,转铁蛋白Tf发挥着决定性的作用,其通过协调多种蛋白来实现铁离子平衡。TF还与一些细胞生长分化有关,包括营养肌肉、胚胎形成、细胞增殖、有丝分裂、趋化和血管形成等等 [8-10]。
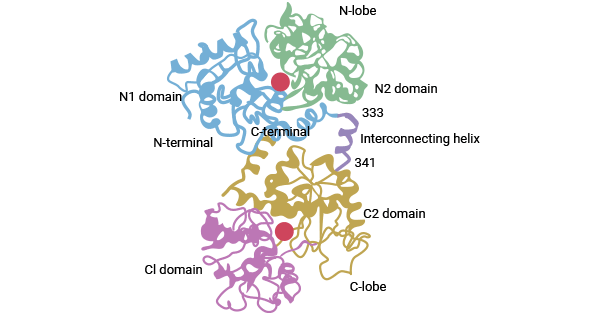
图1. Tf结构示意图 [2]
2. 血清转铁蛋白TF相关的作用机制是什么?
2.1 TF通过受体介导细胞内吞摄取铁离子
TF能与细胞膜上的转铁蛋白受体(Transferrin Receptor,TFR)相结合,通过细胞内吞作用,使有铁的TF-TFR复合体进入细胞。载有铁的TF-TFR复合体内化后变为内含体,由于内含体膜上质子泵介导的pH下降,Tf的构像发生变化,导致Fe3+从TF上释放,在内含体内的铁还原酶催化下使Fe3+转变为Fe2+。然后,在二价金属离子转运体(Divalent metal transporter 1,DMT1)的作用下,Fe2+进入细胞质与铁蛋白(Ferritin heavy chain 1,FTH1)结合储存。Tf-TFR复合体通过受体介导的内吞是细胞摄取铁的主要方式,这一途径不仅有效用于运送抗癌药物和蛋白,还可将治疗基因药物传递到过表达TR的肿瘤细胞(图2) [11-12]。
(您可浏览相关文章:转铁蛋白受体TFR1(TFRC):铁稳态关键成员,贫血、神经退行性疾病、癌症新锐靶点!)
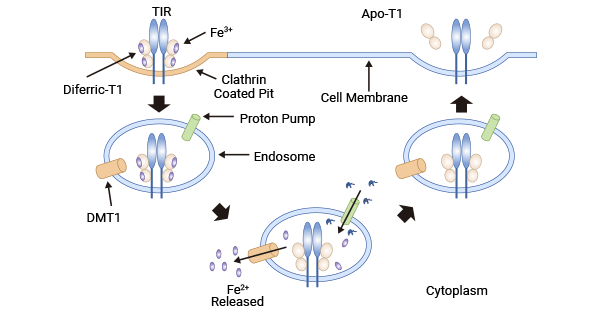
图2. TF通过受体介导细胞内吞摄取铁离子 [11]
2.2 TF促肿瘤细胞对铁死亡的敏感性
铁代谢在细胞铁死亡中扮演关键角色,过多的游离铁离子可能引发自由基产生,导致氧化应激、DNA损伤和脂质过氧化等,最终导致细胞死亡。一项研究调查了一组骨髓瘤患者其外周血标本中与铁代谢相关的指标,尽管铁含量正常,但转铁蛋白减少、铁蛋白增多。转铁蛋白减少提示细胞内铁降低,而铁蛋白增多表明细胞内铁以无毒方式储存。此外,研究发现通过增加转铁蛋白含量,再使用Erastin处理骨髓瘤MM细胞时,转铁蛋白显著提高了细胞对Erastin的敏感性,显著降低了MM细胞增殖能力,且这种敏感性与细胞内铁含量正相关。因此,提高转铁蛋白的含量有助于促进MM细胞对Erastin诱导的铁死亡的易感性 [13-14]。
3. TF和疾病相关的研究
近年来,TF常常被报道用于肿瘤靶向治疗,尤其是Tf修饰的纳米药物被广泛应用于药物释放体系(Drug Delivery System,DDS)。DDS是指利用高分子聚合物、脂质体、外泌体和金属氧化物等纳米材料作为药物载体制成的药物制剂,能够控制药物在人体内环境中持续释放。这使得药物能够在目标组织或病区维持一定时间,为疾病治疗提供更有效的研究手段。
3.1 TF和肝癌研究
一项研究发现,采用Transferrin(Tf)修饰的阿霉素脂质体(Tf-SL-DOX),Tf-SL-DOX可使靶器官的阿霉素浓度明显增高。体外细胞毒实验结果表明,与阿霉素脂质体(SL-DOX)相比,Tf-SL-DOX的IC50为20.4μmol/L,SL-DOX的IC50为166.2μmol/L,两者相比有显著性差异(P<0.01),SL-DOX对体外HepG2肝癌细胞有高效杀伤作用。尾静脉注射Tf-SL-DOX,肿瘤生长速度较模型组明显减慢,抑瘤率达67%,证明Tf-SL-DOX能显著抑制肿瘤组织的生长,提示Tf-SL-DOX对肝癌细胞具有较好的靶向作用和较低的心脏毒副作用 [15-18]。
3.2 TF和乳腺癌研究
在绿色荧光发射碳点GCD上引入TF修饰,将阿霉素DOX与GCD结合形成GCD-PEG-Tf@DOX。用GCD-PEG-Tf@DOX处理乳腺癌细胞MCF-7时,其通过靶向TR过表达的癌细胞实现精准给药。共聚焦显微镜显示,在TfR表达较高的MCF-7细胞中,GCD-PEG-Tf@DOX荧光强,而在TfR表达较低的CHO细胞中荧光较弱。因此,GCD-PEG-Tf@DOX能准确靶向TfR过表达的乳腺癌细胞。细胞毒性实验证明,GCD-PEG-Tf@DOX的细胞毒性具有时间和浓度依赖性,处理后的细胞状态与游离的DOX相似,说明GCD-PEG-Tf@DOX不会降低DOX的细胞毒性。向肿瘤小鼠注射GCD-PEG-Tf@DOX,发现Tf纳米药物显著抑制肿瘤生长,小鼠体重无明显变化,表明GCD-PEG-Tf@DOX副作用小且具有良好的靶向能力 [19-22]。
3.3 TF和前列腺癌研究
一项研究分析了转铁蛋白、clusterin和转甲状腺素蛋白在前列腺正常、增生、癌组织中的表达和意义。结果显示,Tf在前列腺癌组织中的表达明显高于良性前列腺增生组和正常前列腺组。在前列腺癌组中阳性达94%。进一步研究发现,其表达阳性率与前列腺癌病理分级和临床分期均呈线性正相关,提示Tf在前列腺癌组织中同样有高表达且阳性率较高,与Tf在血清中的表达结果一致。因此,研究者认为Tf是前列腺癌血清中差异表达蛋白质,其参与前列腺癌的发展过程可以作为病情进展和评价治疗的指标 [23-25]。
3.4 TF和其它肿瘤等疾病研究
一些研究报道表明,在神经胶质瘤、肺腺癌、卵巢癌、慢性淋巴细胞白血病等恶性程度高及易转移的肿瘤中,Tf也存在异常表达 [26-29]。例如,研究者发现HP+Tf+CEA联合运用,能提高早期卵巢上皮癌诊断特异性和灵敏度,有助于提高CA125阴性卵巢上皮癌的检出率 [30]。此外,在一项研究中,研究者设计了Tf-5-ALA-PTX-NCs纳米平台,结合了化学和光动力治疗,通过磁靶向效应和TR的主动靶向,提高了肿瘤靶向能力。实验证明,该平台在体内外实现了肿瘤特异性靶向和协同治疗的抗肿瘤效果 [31]。
腹膜透析相关性腹膜炎(PDAP)研究发现,PDAP患者普遍存在铁代谢紊乱,且血清铁、铁蛋白、转铁蛋白是PDAP发生的危险因素 [32]。另外,低水平的转铁蛋白与贫血发病率增加相关 [33]。在感染时,TF通过形成转铁蛋白螯合复合物抑制细菌、病毒及真菌的生长,为抗感染药物研究提供新方向 [34]。
4. TF的临床药物研究前景
转铁蛋白TF作为一种特异性结合并转运铁的糖蛋白,在临床药物研究中呈现广泛前景。以往报道表明,TF可作为肿瘤治疗的靶向配体,实现肿瘤特异性治疗,还结合协同化疗和光动力疗法显著抑制肿瘤生长,降低毒副作用。此外,TF修饰的脂质体作为改进的药物载体,通过携带逆转多药耐药的药物和抗肿瘤药物实现靶向输送,提高药物浓度,减少正常组织毒副作用。TF还能抑制微生物生长为抗感染药物提供新方向。同时,低水平的转铁蛋白与贫血发病率增加相关。总体而言,TF的研究为抗感染,尤其是肿瘤方面的研究提供了多层面的应用前景。
为鼎力协助各药企针对血清转铁蛋白TF在抗感染、贫血、肿瘤等疾病在临床中的研究,j9九游会登录入口首页CUSABIO推出血清转铁蛋白TF(CSB-MP023412HU)活性蛋白产品,助力您在对血清转铁蛋白TF机制方面的研究或其潜在临床价值的探索。
j9九游会登录入口首页 血清转铁蛋白Serotransferrin(TF)
Recombinant Human Serotransferrin(TF) (Active) Code: CSB-MP023412HU
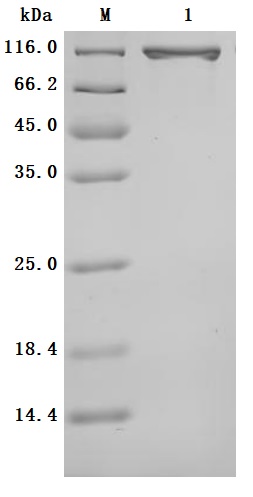
Purity was greater than 95% as determined by SDS-PAGE.
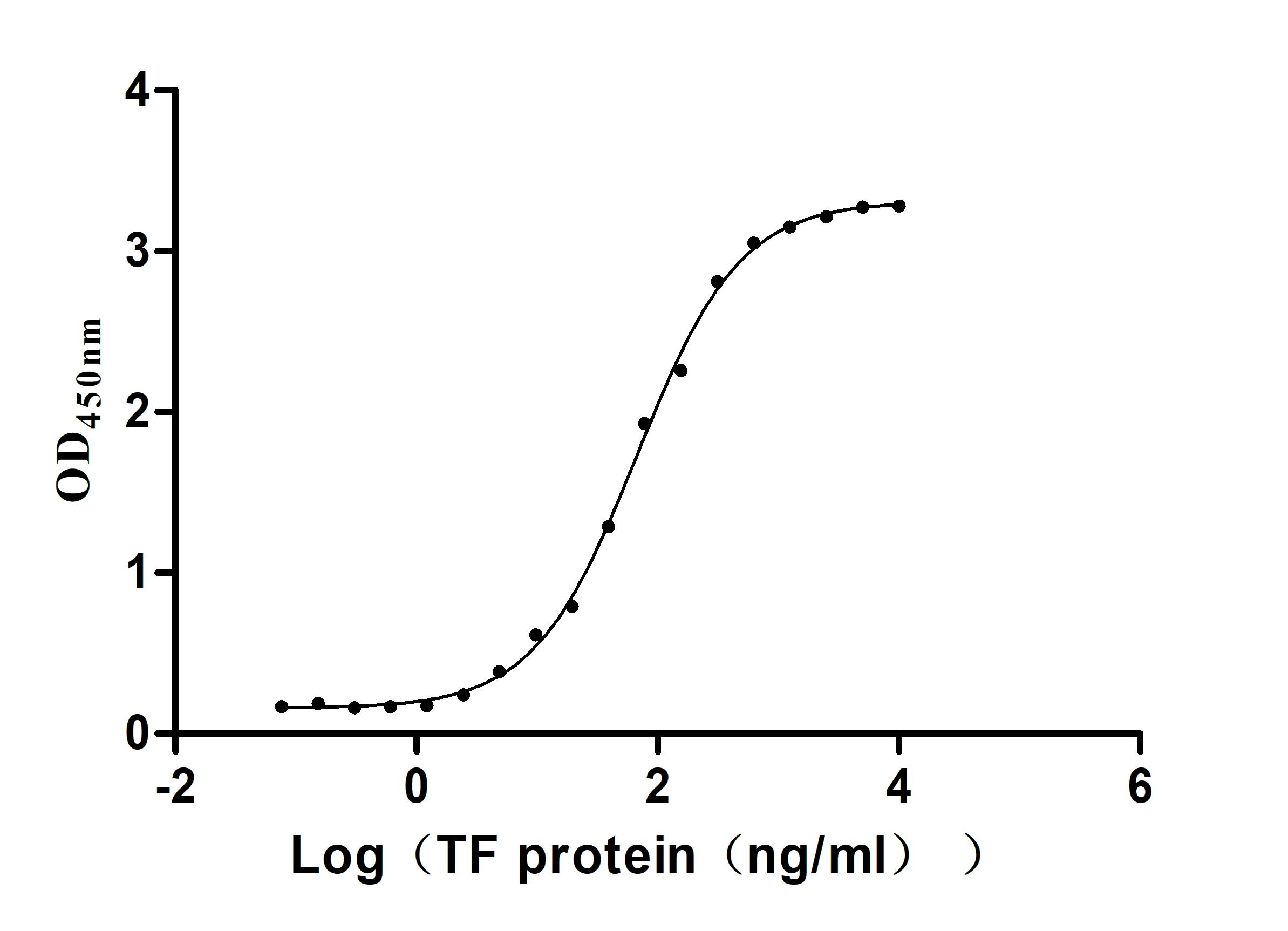
Immobilized TFRC(CSB-MP3648HU)at 2μg/mL can bind Human TF. The EC50 is 58.72-77.84 ng/mL.
参考文献:
[1] Mattera, Vanesa, et al. "Remyelinating effect driven by transferrin‐loaded extracellular vesicles." Glia 72.2 (2024): 338-361.
[2] Koneru, Tejaswi, et al. "Transferrin: biology and use in receptor-targeted nanotherapy of gliomas." ACS omega 6.13 (2021): 8727-8733.
[3] Chasteen, N. Dennis. "Human serotransferrin: structure and function." Coordination Chemistry Reviews 22.1-2 (1977): 1-36.
[4] Kimawaha, Phongsaran, et al. "Serum α2, 6-sialylated glycoform of serotransferrin as a glycobiomarker for diagnosis and prediction of clinical severity in cholangiocarcinoma." Clinica Chimica Acta 536 (2022): 142-154.
[5] Barik, Sushanta Kumar, et al. "Identification and differential expression of serotransferrin and apolipoprotein AI in the plasma of HIV-1 patients treated with first-line antiretroviral therapy." BMC infectious diseases 20 (2020): 1-8.
[6] Lai, Ren, et al. "Transferrin is upregulated by microbes and acts as a negative regulator of immunity to induce intestinal immunotolerance." Research (2024).
[7] Zakin, Mario M. "Regulation of transferrin gene expression." The FASEB journal 6.14 (1992): 3253-3258.
[8] Lok, C. N., and T. T. Loh. "Regulation of transferrin function and expression: review and update." Neurosignals 7.3 (1998): 157-178.
[9] Ogun, Aminat S., and Adebayo Adeyinka. "Biochemistry, transferrin." StatPearls [Internet]. StatPearls Publishing, 2022.
[10] Silva, André MN, et al. "Human transferrin: An inorganic biochemistry perspective." Coordination Chemistry Reviews 449 (2021): 214186.
[11] Daniels TR, Delgado T, Helguera G, Penichet ML. The transferrin receptor part II: targeted delivery of therapeutic agents into cancer cells. Clin Immunol. 2006 Nov;121(2):159-76.
[12] Candelaria, Pierre V., et al. "Antibodies targeting the transferrin receptor 1 (TfR1) as direct anti-cancer agents." Frontiers in immunology 12 (2021): 607692.
[13] Li, Jiaojiao, and Wei Zhang. "From iron chelation to overload as a therapeutic strategy to induce ferroptosis in hematologic malignancies." Hematology 27.1 (2022): 1163-1170.
[14] Zhao, Yan, Zineng Huang, and Hongling Peng. "Molecular mechanisms of ferroptosis and its roles in hematologic malignancies." Frontiers in oncology 11 (2021): 743006.
[15] R Nogueira-Librelotto, Daniele, et al. "Transferrin-conjugated nanocarriers as active-targeted drug delivery platforms for cancer therapy." Current pharmaceutical design 23.3 (2017): 454-466.
[16] Li, XueMing, et al. "Targeted delivery of doxorubicin using stealth liposomes modified with transferrin." International journal of pharmaceutics 373.1-2 (2009): 116-123.
[17] Sen, Kacoli, and Mahitosh Mandal. "Second generation liposomal cancer therapeutics: transition from laboratory to clinic." International journal of pharmaceutics 448.1 (2013): 28-43.
[18] Huang, Shan-Zhou, et al. "Targeting TF-AKT/ERK-EGFR pathway suppresses the growth of hepatocellular carcinoma." Frontiers in oncology 9 (2019): 150.
[19] Li, Lihong, et al. "Targeted delivery of doxorubicin using transferrin-conjugated carbon dots for cancer therapy." ACS Applied Bio Materials 4.9 (2021): 7280-7289.
[20] Kölbl, Alexandra C., et al. "The role of TF-and Tn-antigens in breast cancer metastasis." (2016).
[21] Zhou, Jun, et al. "A transferrin-conjugated hollow nanoplatform for redox-controlled and targeted chemotherapy of tumor with reduced inflammatory reactions." Theranostics 8.2 (2018): 518.
[22] Mahani, Mohamad, et al. "Doxorubicin delivery to breast cancer cells with transferrin-targeted carbon quantum dots: An in vitro and in silico study." Journal of Drug Delivery Science and Technology 62 (2021): 102342.
[23] Ye, Yun, Su-Liang Li, and Sheng-Yu Wang. "Construction and analysis of mRNA, miRNA, lncRNA, and TF regulatory networks reveal the key genes associated with prostate cancer." PloS one 13.8 (2018): e0198055.
[24] Al Robaian, Majed, et al. "Therapeutic efficacy of intravenously administered transferrin-conjugated dendriplexes on prostate carcinomas." Nanomedicine 9.4 (2014): 421-434.
[25] Fernandes, Mariza Aires, et al. "Transferrin-functionalized liposomes for docetaxel delivery to prostate cancer cells." Colloids and Surfaces A: Physicochemical and Engineering Aspects 611 (2021): 125806.
[26] Dufès, Christine, Majed Al Robaian, and Sukrut Somani. "Transferrin and the transferrin receptor for the targeted delivery of therapeutic agents to the brain and cancer cells." Therapeutic delivery 4.5 (2013): 629-640.
[27] Wu, Yihe, et al. "Blocking transferrin receptor inhibits the growth of lung adenocarcinoma cells in vitro." Thoracic Cancer 9.2 (2018): 253-261.
[28] Deshpande, Pranali, et al. "Transferrin and octaarginine modified dual-functional liposomes with improved cancer cell targeting and enhanced intracellular delivery for the treatment of ovarian cancer." Drug delivery 25.1 (2018): 517-532.
[29] Metzgeroth, Georgia, et al. "The soluble transferrin receptor reflects tumor load in chronic lymphocytic leukemia." (2007): 1313-1318.
[30] Kobayashi, Eiji, et al. "Biomarkers for screening, diagnosis, and monitoring of ovarian cancer." Cancer Epidemiology, Biomarkers & Prevention 21.11 (2012): 1902-1912.
[31] Ge, Pingyun, et al. "Transferrin receptors/magnetic resonance dual-targeted nanoplatform for precise chemo-photodynamic synergistic cancer therapy." Nanomedicine: Nanotechnology, Biology and Medicine 39 (2022): 102467.
[32] Liu, Xiaoli, et al. "Therapeutic applications of multifunctional nanozymes." Nanoscale 11.44 (2019): 21046-21060.
[33] Boshuizen, M., et al. "Therapeutic use of transferrin to modulate anemia and conditions of iron toxicity." Blood Reviews 31.6 (2017): 400-405.
[34] Bruhn, Kevin W., and Brad Spellberg. "Transferrin-mediated iron sequestration as a novel therapy for bacterial and fungal infections." Current opinion in microbiology 27 (2015): 57-61.











