MYL9:肌球蛋白“细胞动力马达”家族一员,癌细胞迁移动力靶点?
日期:2023-07-12 17:15:38
MYL9作为肌球蛋白的调节性轻链(myosin light chain,MLC),是激活肌球蛋白运动活性的关键环节,为细胞运动提供动力。然而,细胞迁移是造成恶性肿瘤转移的关键。多项研究表明,MYL9为癌细胞迁移提供动力,参与肿瘤的发生、发展。CD69被认为是淋巴细胞的早期激活标志物,最新研究阐明了一种新机制,MYL9可以作为CD69的配体,参与T细胞募集到炎症性肺部 [1]。
此外,MYL9-CD69系统可诱导肿瘤微环境中的效应T细胞衰竭,并削弱抗肿瘤免疫反应。通过阻断MYL9和CD69之间的相互作用,有利于保持较高的抗肿瘤免疫反应 [2]。因此,MYL9作为肌球蛋白“细胞动力马达”家族一员,可能是癌症免疫治疗的下一个新药物靶点!
1. 什么是肌球蛋白?
肌球蛋白是一类高度保守的蛋白,是细胞骨架的重要组成部分,也是基于肌动蛋白依赖性的分子马达。肌球蛋白约占人体总蛋白的15%~25%,主要存在平滑肌中,其家族比较大,目前已发现人40个肌球蛋白基因,分别代表12类的超大家族(I-XII)。肌球蛋白的分子结构包括重链(myosin heavy chain,MHC)和轻链(myosin light chain,MLC),轻链又分为基础性MLC(MLC I)和调节性MLC(MLC II)。肌球蛋白是一种多功能蛋白质,在细胞的变形、黏附、运动及细胞的迁移方面有巨大的作用,被称为“细胞动力的马达”(图1) [3-6]。
2. 什么是MYL9?
肌球蛋白监管轻链MYL9(myosin regulatory light chain 9,又称MLC2, MRLC1或MLC-2C)是组成肌球蛋白的调节性轻链。人MYL9基因位于染色体20q11.23上,包含8621个碱基,8个内含子和17个外显子。它编码的20KD肌球蛋白监管轻链,是肌球蛋白的重要组成部分。肌球蛋白按区域分为头部、颈部和尾部。头部是球形,含有ATP结合和肌动蛋白结合部位,用于催化ATP的水解释放化学能量,并与肌动蛋白结合。颈部有两条调节性轻链缠绕成α螺旋状,可结合肌球蛋白轻链和钙调素。尾部是卷曲的螺旋结构,含有羟基 (图2)[7]。
MYL9在正常组织及肿瘤组织中均广泛表达,如肌肉组织、内脏组织、肺癌、前列腺癌等。迄今为止,已有大量研究表明,MYL9作为肌球蛋白的关键成员,通过多种调节因子参与生物体内诸多的功能,如肌肉运动,细胞迁移,细胞内吞,有丝分裂和信号转导等,尤其在肿瘤细胞的迁移和增殖中发挥着重要的作用 [8-11]。
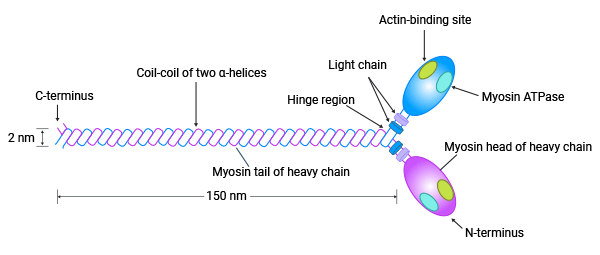
图2. MYL9是肌球蛋白“细胞动力马达”关键成员 [7]
3. MYL9相关的信号通路
MYL9主要通过两大系统起到调控的作用:1)Rho肌酶(rho-kinase, ROCK)系统;2)肌球蛋白轻链肌酶(myosin light chain kinase, MLCK)系统。除了这两个系统之外,还涉及钙调蛋白依赖的蛋白激酶II、ILK、PKA、ZIPK、PKC以及肌球蛋白轻链磷酸酶MLCP抑制分子CP17等相关重要分子 [12-14]。
如图3所示,MLCK和ROCK系统激活,并与钙离子Ca2+结合,增加细胞内钙离子Ca2+浓度,又可以激活肌球蛋白轻链肌酶活性和MYL9的磷酸化。具体而言,ROCK是肌球蛋白轻链的最直接底物之一,其介导MYL9基因的磷酸化及去磷酸化作用。此外,ROCK可促进细胞内Ca2+浓度的增加,通过激活Ca2+/钙调蛋白依赖的MLCK,在MLCK的作用下引起MYL9的磷酸化 [12]。
MYL9的磷酸化增强肌球蛋白和肌动蛋白之间的相互作用,以及肌球蛋白轻链头部ATP酶的活性,从而促进细胞骨架的重构,并增加细胞的增殖、分化、粘附和迁移的能力。RhoA抑制因子能有效的直接抑制其下游的效应蛋白Rock,从而对MYL9的表达和磷酸化作用起抑制作用,进而抑制肿瘤细胞肌动蛋白形成等生物学行为,同时对肿瘤的侵袭、转移和生长也起抑制作用 [12]。
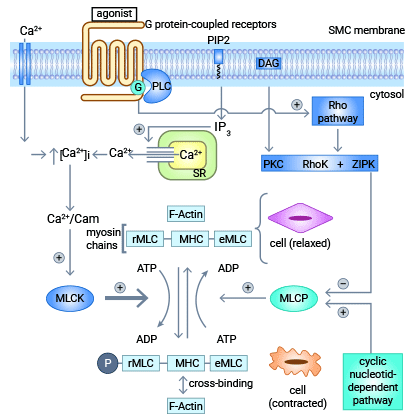
图3. MYL9主要通过MLCK和ROCK两大系统起到调控的作用 [12]
4. MYL9在肿瘤等疾病中的作用
许多研究表明,MYL9通过磷酸化和去磷酸化调节ATP酶活性以及肌球蛋白的收缩,从而控制细胞的运动、黏附和变形等功能。恶性肿瘤的特点之一是浸润性生长和远处转移,这表明与细胞运动相关的分子MYL9异常表达可能影响肿瘤的发展。许多研究已经报道了MYL9参与肿瘤发病机制,不仅能抑制肿瘤,同时也能促进肿瘤生长 [15-17]。
4.1 MYL9和肿瘤
4.1.1 MYL9促进肿瘤
MYL9在多种肿瘤中呈现增加的表达和磷酸化水平。在乳腺癌和肝癌中,MYL9主要通过促进肿瘤细胞运动来促使肿瘤发生侵袭 [18-19]。此外,在胶质母细胞瘤中,MYL9的表达量和磷酸化水平也有所增加。高水平的MYL9表达与胶质母细胞瘤患者预后较差以及复发性胶质母细胞瘤患者中MYL9表达增加相关联 [8]。另外,胰腺导管腺癌和卵巢上皮性肿瘤中也观察到MYL9的增加表达。临床数据分析提示MYL9可作为胰腺导管腺癌和卵巢上皮性肿瘤的独立预后因素 [14, 20]。
同样地,食管鳞状细胞癌细胞系中MYL9呈显著上调,并且与肿瘤细胞中MYL9表达高的患者总生存期和无复发生存期较差相关 [21]。此外,高表达MYL9促进骨肉瘤的进展 [22]。综上所述,这些研究结果表明MYL9可能通过促进肿瘤细胞运动影响肿瘤的生长和侵袭转移,并且可能成为多种肿瘤的预后标志物和治疗靶点。
4.1.2 MYL9抑制肿瘤
但与之相反的是,基于微阵列和蛋白质组学分析,发现MYL9蛋白在前列腺组织中表达下降,与年龄、病理分期、转移和PSA水平等相关 [23]。类似地,在膀胱癌和胃癌中也观察到MYL9表达下调 [24-25]。然而,进一步的细胞实验证明,MYL9缺乏减少了胃癌细胞的增殖并增强了细胞凋亡 [15]。在人类结直肠癌中,MYL9的表达和磷酸化水平降低 [26]。细胞实验证实,通过上调MYL9,可以抑制肿瘤细胞的增殖、侵袭和迁移,并促进结肠癌干细胞的凋亡 [27-28]。
生物信息学分析发现,低MYL9表达可能与非小细胞肺癌的发展和转移有关 [29-30]。在乳腺癌中,MYL9的上调显著降低了癌细胞的运动能力 [15]。然而,一些文献提出MYL9在分子水平上的表达增加了乳腺癌细胞的迁移 [15, 31]。根据目前的文献,MYL9在恶性肿瘤中的作用存在争议。总体而言,MYH9在肿瘤中的作用复杂多样,可能成为治疗靶点和预后评估的重要指标。
4.2 MYL9和其它疾病
MYL9不仅与肿瘤相关,还在心血管、炎症和神经系统等疾病中发挥作用 [32-35]。动脉粥样硬化模型显示,随着斑块增加,MYL9表达显著下调。MYL9受到血管紧张素II(Angiotensin II/AGT)和细胞因子PDGF-BB的刺激下调。血管紧张素II和PDGF-BB是重要的细胞因子,在血管损伤过程中促进平滑肌细胞增殖和迁移。因此,MYL9可能在动脉粥样硬化的发展中起重要作用 [36-37]。
陆续的报道表明MYL9蛋白是CD69分子的新配基,MYL9-CD69系统参与调节免疫应答 [2, 38-41]。阻断MYL9-CD69之间的互动可改善实验鼠的过敏性呼吸道炎症,如哮喘 [2];在炎症时,血管受损并导致血小板活化,进而产生MYL9网络结构和各种凝血因子,接着MYL9能够与CD69阳性的特异性T细胞结合,促使免疫细胞聚集到炎症部位,并产生效应细胞因子和趋化因子,从而有效激活免疫反应(图4) [41]。
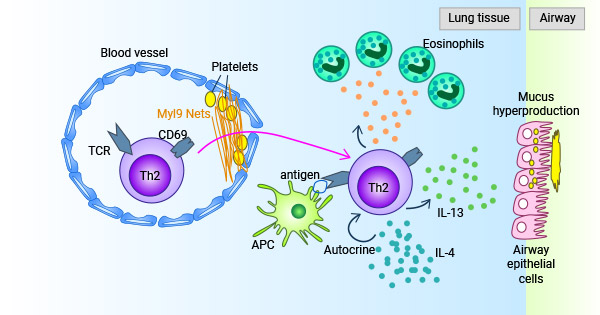
图4. MYL9-CD69系统参与调节免疫应答 [42]
5. MYL9的临床研究前景
MYL9在肺癌、乳腺癌、前列腺癌、胃癌等多种不同类型的恶性肿瘤中均有异常表达,并且往往与不良预后密切相关,其表达的临床意义根据癌组织的不同而不同。更值得关注的是,MYL9可能作为CD69新配体,两者的相互作用促进CTL的滞留,使其受到肿瘤抗原的慢性刺激而导致T细胞衰竭。在肿瘤微环境中,抗原特异性细胞毒性T细胞(cytotoxic lymphocytes,CTL)是机体抗肿瘤免疫的重要防线。这意味着抗MYL9/CD69可能是癌症免疫疗法的理想治疗靶点。
目前,国内外针对MYL9抗癌的临床实践经验尚浅,但其作为各类肿瘤治疗的靶标的可能性已得到了越来越多的证据支持。因此,MYL9作为分子标志物和潜在的靶标,在恶性肿瘤的早期诊断、预后预测以及靶向治疗中将具有极大的临床应用价值。
为鼎力协助科研和药企人员针对MYL9在肿瘤等疾病中的临床应用研究,CUSABIO推出MYL9活性蛋白(Code: CSB-YP015318HU)产品,助力您在MYL9机制方面的研究或其潜在临床价值的探索。(点击查看MYL9系列产品: MYL9蛋白; MYL9抗体)
Recombinant Human Myosin regulatory light polypeptide 9(MYL9) (Active) (Code: CSB-YP015318HU)
The Greater than 95% as determined by SDS-PAGE. (Tris-Glycine gel) Discontinuous SDS-PAGE (reduced) with 5% enrichment gel and 15% separation gel.
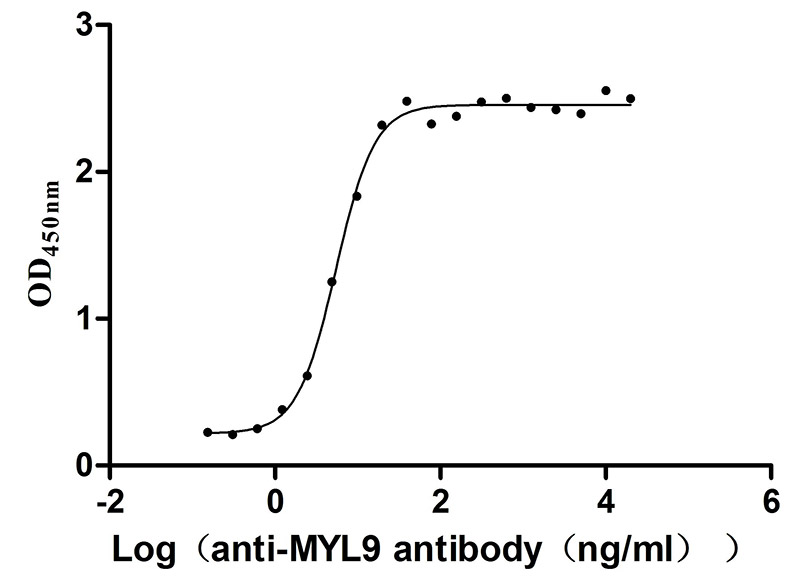
Immobilized Human MYL9 at 2μg/mL can bind Anti-MYL9 recombinant antibody (CSB-RA015318MA1HU), the EC50 is 4.628-6.430 ng/mL.
参考文献:
[1] Iwamura, Chiaki, et al. "Elevated Myl9 reflects the Myl9-containing microthrombi in SARS-CoV-2-induced lung exudative vasculitis and predicts COVID-19 severity." Proceedings of the National Academy of Sciences 119.33 (2022): e2203437119.
[2] Kimura, Motoko Y., et al. "A new therapeutic target: the CD69-Myl9 system in immune responses." Seminars in immunopathology. vol. 41. Springer Berlin Heidelberg, 2019.
[3] Sellers, James R. "Myosins: a diverse superfamily." Biochimica et Biophysica Acta (BBA)-Molecular Cell Research 1496.1 (2000): 3-22.
[4] Fitz, Gillian N., et al. "Protrusion growth driven by myosin-generated force." Developmental Cell 58.1 (2023): 18-33.
[5] Hartman, M. Amanda, and James A. Spudich. "The myosin superfamily at a glance. "Journal of cell science 125.7 (2012): 1627-1632.
[6] Lewis, Christopher TA, and Julien Ochala. "Myosin heavy chain as a novel key modulator of striated muscle resting state. "Physiology 38.1 (2023): 3-9.
[7] Bhanu P. Jena. "Myosin: Cellular Molecular Motor" Cellular Nanomachines (2020): 79-89.
[8] Kruthika, Banavathy S., et al. "Expression pattern and prognostic significance of myosin light chain 9 (MYL9): a novel biomarker in glioblastoma." Journal of Clinical Pathology 72.10 (2019): 677-681.
[9] Shehadeh, Lina A., et al. "Dynamic regulation of vascular myosin light chain (MYL9) with injury and aging." PLoS One 6.10 (2011): e25855.
[10] Zhu, Kongxi, et al. "Long non-coding RNA MBNL1-AS1 regulates proliferation, migration, and invasion of cancer stem cells in colon cancer by interacting with MYL9 via sponging microRNA-412-3p." Clinics and research in hepatology and gastroenterology 44.1 (2020): 101-114.
[11] Jalagadugula, Gauthami, et al. "Regulation of platelet myosin light chain (MYL9) by RUNX1: implications for thrombocytopenia and platelet dysfunction in RUNX1 haplodeficiency." Blood, The Journal of the American Society of Hematology 116.26 (2010): 6037-6045.
[12] Moreno, Carolina Araujo, et al. "Homozygous deletion in MYL9 expands the molecular basis of megacystis-microcolon- intestinal hypoperistalsis syndrome." European Journal of Human Genetics 26.5 (2018): 669-675.
[13] Licht, Alexander H., et al. "Junb regulates arterial contraction capacity, cellular contractility, and motility via its target Myl9 in mice." The Journal of clinical investigation 120.7 (2010): 2307-2318.
[14] Matsushita, Katsunori, et al. "Clinicopathological significance of MYL9 expression in pancreatic ductal adenocarcinoma." Cancer Reports 5.10 (2022 ): e1582.
[15] Lv, Minghe, Lumeng Luo, and Xue Chen. "The landscape of prognostic and immunological role of myosin light chain 9 (MYL9) in human tumors." Immunity. Inflammation and Disease 10.2 (2022): 241-254.
[16] Gilles, Laure, et al. "MAL/SRF complex is involved in platelet formation and megakaryocyte migration by regulating MYL9 (MLC2) and MMP9." Blood, The Journal of the American Society of Hematology 114.19 (2009): 4221-4232.
[17] Tan, Xiang, and Mingwu Chen. "MYLK and MYL9 expression in non-small cell lung cancer identified by bioinformatics analysis of public expression data." Tumor Biology 35 (2014): 12189-12200.
[18] Luo, Xue-Gang, et al. "Histone methyltransferase SMYD3 promotes MRTF-A-mediated transactivation of MYL9 and migration of MCF-7 breast cancer cells." Cancer letters 344.1 (2014): 129-137.
[19] Hirasawa, Yuichi, et al. "Methylation status of genes upregulated by demethylating agent 5-aza-2′-deoxycytidine in hepatocellular carcinoma." Oncology 71.1-2 (2007): 77-85.
[20] Deng, Yuao, et al. "High expression of MYL9 indicates poor clinical prognosis of epithelial ovarian cancer." Recent Patents on Anti-Cancer Drug Discovery 16.4 (2021): 533-539.
[21] Wang, Jian-Hua, et al. "Expression and prognostic significance of MYL9 in esophageal squamous cell carcinoma." ploS one 12.4 (2017): e0175280.
[22] Zhao, S., W. Xiong, and K. Xu. "MiR-663a, regulated by lncRNA GAS5, contributes to osteosarcoma development through targeting MYL9. "Human & Experimental Toxicology 39.12 (2020): 1607-1618.
[23] Huang, Ya-qiang, et al. "Decreased expression of myosin light chain MYL9 in stroma predicts malignant progression and poor biochemical recurrence- free survival in prostate cancer." medical oncology 31 (2014): 1-9.
[24] Huang, Chu-Han, et al. "MYL9 deficiency is neonatal lethal in mice due to abnormalities in the lung and the muscularis propria of the bladder and intestine ." Plos one 17.7 (2022): e0270820.
[25] Dong, Ningxin, et al. "Identification and validation of critical genes with prognostic value in gastric cancer." Frontiers in Cell and Developmental Biology 10 (2022): 1072062.
[26] Qiu, Xiao, et al. "Weighted gene co-expression network analysis identified MYL9 and CNN1 are associated with recurrence in colorectal cancer." journal of Cancer 11.8 (2020): 2348.
[27] Zhou, Rui, et al. "PRPF19 facilitates colorectal cancer liver metastasis through activation of the Src-YAP1 pathway via K63-linked ubiquitination of MYL9." Cell Death & Disease 14.4 (2023): 258.
[28] Feng, Min, et al. "Myosin light chain 9 promotes the proliferation, invasion, migration and angiogenesis of colorectal cancer cells by binding to Yes- associated protein 1 and regulating Hippo signaling." Bioengineered 13.1 (2022): 96-106.
[29] Sheng, Meiling, Zhaohui Dong, and Yanping Xie. "Identification of tumor-educated platelet biomarkers of non-small-cell lung cancer. "OncoTargets and therapy (2018): 8143-8151.
[30] Tan, Xiang, and Mingwu Chen. "MYLK and MYL9 expression in non-small cell lung cancer identified by bioinformatics analysis of public expression data." Tumor Biology 35 (2014): 12189-12200.
[31] Zhang, Chunling, et al. "Myocardin-related transcription factor A is up-regulated by 17β-estradiol and promotes migration of MCF-7 breast cancer cells via transactivation of MYL9 and CYR61." Acta Biochim Biophys Sin 45.11 (2013): 921-927.
[32] Kandler, Justin L., et al. "Compound heterozygous loss of function variants in MYL9 in a child with megacystis-microcolon intestinal hypoperistalsis syndrome." Molecular Genetics & Genomic Medicine 8.11 (2020): e1516.
[33] Kobayashi, Hironobu, et al. "Increased Myosin light chain 9 expression during Kawasaki disease vasculitis." Frontiers in Immunology 13 (2023). 1036672.
[34] Xiong, Yao, et al. "Targeting MRTF/SRF in CAP2-dependent dilated cardiomyopathy delays disease onset." jci insight 4.6 (2019).
[35] Sun, L., et al. "Decreased platelet expression of myosin regulatory light chain polypeptide (MYL9) and other genes with platelet dysfunction and CBFA2/ RUNX1 mutation: insights from platelet expression profiling." Journal of Thrombosis and Haemostasis 5.1 (2007): 146-154.
[36] Wen, Dingke, et al. "Single-cell RNA sequencing reveals the pathogenic relevance of intracranial atherosclerosis in blood blister-like aneurysms." Frontiers in Immunology 13 (2022): 927125.
[37] Hwang, Ki-Chul. "The Role of MicroRNAs in Vascular Diseases; Smooth Muscle Cell Differentiation and De-Differentiation. "Korean Circulation Journal 44.4 (2014): 218-219.
[38] Hayashizaki, Koji, et al. "Myosin light chains 9 and 12 are functional ligands for CD69 that regulate airway inflammation." science immunology 1.3 (2016 ): eaaf9154-eaaf9154.
[39] Nakayama, Toshinori, et al. "CD4+ T cells in inflammatory diseases: pathogenic T-helper cells and the CD69-Myl9 system." International Immunology 33.12 (2021): 699-704.
[40] Yokoyama, Masaya, et al. "Myosin light chain 9/12 regulates the pathogenesis of inflammatory bowel disease." Frontiers in Immunology 11 (2021): 594297.
[41] Onodera, Atsushi, Kota Kokubo, and Toshinori Nakayama. "Epigenetic and transcriptional regulation in the induction, maintenance, heterogeneity and recall-response of effector and memory Th2 cells." Frontiers in Immunology 9 (2018): 2929.