肝肠钙粘蛋白CDH17:单抗、双抗、CAR T“多管齐下”,胃肠道癌GIC重磅靶点!
日期:2023-03-27 14:58:09
近年来,胃肠道癌(Gastrointestinal carcinoma, GIC)的靶向治疗逐渐成为研究的热点,越来越多的靶点被发现,如EGFR、HER-2、PD-1、CTLA-4、VEGF、DDR1、LAG-3、MUC1、TIGIT、CLDN18.2等。新型靶向治疗药物还在不断涌现,美国制药巨头勃林格殷格翰Boehringer Ingelheim近期开发了一款创新型基于CDH17的双特异性抗体CDH17/TRAILR2/(BI 905711),已处于I/II期临床阶段,用于胃癌、胰腺癌、食管癌、胆管癌等胃肠道癌。BI 905711可同时结合肿瘤细胞表面的CDH17和死亡受体TRAILR2,诱导肿瘤细胞凋亡,对表达CDH17的正常细胞无损伤。
此外,香港生物制药公司Arbele的CDH17单抗(ARB102)和双特异性抗体CDH17/CD3(ARB202),正处于临床前研究阶段,用于治疗胰腺癌、胆管癌等实体瘤。另有研究报道,一款针对CDH17的VHH1-CAR T细胞治疗,可特异性根除表达CDH17的神经内分泌肿瘤NET、胃癌、胰腺癌以及结直肠癌细胞。因此,CDH17作为钙黏蛋白超家族的新成员,成为开发更安全的实体瘤免疫疗法的重磅靶点,尤其是胃肠道癌!
1. 什么是钙黏蛋白超家族?
钙黏蛋白(Cadherins)是一类介导钙依赖型细胞间黏附的粘附分子超家族。家族成员众多,根据结构不同可分为经典钙黏蛋白(如E-钙黏蛋白和N-钙黏蛋白)、桥粒钙黏蛋白、原钙黏蛋白、七次跨膜钙黏蛋白和FAT样钙黏蛋白等。它们的主要功能是介导细胞间Ca2+依赖的同型或异型黏附,而且涉及多种信号传导包括Wnt/β-连环蛋白(β-catenin)、PI3K/Akt、Rho GTPase和NF-κB等通路,从而调控相互黏附细胞间的行为 [1-3]。
近年有许多研究表明,钙黏蛋白的敲低或过表与多种疾病有关,如哮喘 [4]、慢性牙周炎 [5],动脉粥样硬化 [6]、糖尿病 [7]等。在一些肿瘤的侵袭和转移过程中,某些黏附分子介导的细胞黏附力降低在肿瘤细胞生物学行为中发挥着关键作用。例如,E-cadherin在多种肿瘤中被认为是抑癌基因,而N-Cadherin作为肿瘤侵袭的启动子,被视为肿瘤细胞获得侵袭性的必要条件 [8-10]。因此,钙黏蛋白或可作为药物靶点,成为治疗过敏、自身免疫病及肿瘤的一种新的手段。
2. 什么是CDH17?
2.1 CDH17的结构
肝肠钙黏蛋白(liver- intestine cadherin, Cadherin-17,又名CDH17;HPT-1;LI-Cadherin)是近年来新发现的一种钙黏蛋白,其最早于1994年由Dietmar等通过分子克隆技术从鼠肝细胞cDNA文库中分析得到的,由于在小鼠仅表达于肝脏和小肠,故命名为肝肠钙黏蛋白 [11]。人CDH17基因定位于染色体8q22. 1,其结构与钙黏蛋白家族成员具有同源性,但CDH17又有以下独特的结构:①CDH17的胞外部分含有7个钙黏蛋白重复区域,而经典钙黏蛋白和桥粒钙黏蛋白为5个;②在CDH17氨基末端的细胞黏附识别区内,决定细胞黏附特性的HAV序列由AAL序列代替;③CDH17细胞质尾仅含有20个氨基酸残基,经典钙黏蛋白有150-160个。因此,CDH17被划分为经典钙黏蛋白的变异体 (图1) [12-14]。
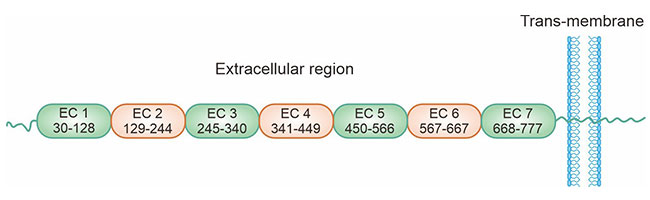
图1. CDH17的结构 [14]
2.2 CDH17的表达及功能
CDH17主要表达于胚胎、成人肠上皮细胞和部分胰腺导管上皮细胞,在健康人群肝细胞、食管上皮细胞及胃黏膜中几乎不表达 [15-18]。CDH17在细胞黏附过程中充当了与钙黏蛋白同样重要的角色,CDH17可直接与细胞支架连接,发挥其细胞黏附作用,而经典的钙黏蛋白必须与链蛋白结合形成复合体才能发挥其黏附功能 [19-20]。在一些病理情况下,CDH17可表达于其它组织。目前研究发现,CDH17在胃癌、结直肠癌、肝癌、胰腺癌和胆管癌等多种肿瘤组织中均有不同程度的表达,CDH17的高水平表达与患者预后和风险评估密切相关 [21-23]。
3. CDH17在肿瘤中的作用机制
CDH17作为钙黏蛋白超家族中的独特一员,在多种疾病中均发现CDH17的异常表达。研究揭示CDH17的功能紊乱与肿瘤细胞的外周浸润及转移关系密切,对肿瘤的复发及患者的生存率均有影响。然而,目前对于CDH17与肿瘤相关作用机制尚未阐明。
在胃癌中,CDH17高表达可使E-cadherin/catenin复合物失去稳定而解离 [23]。研究已证实β-catenin和E-cadherin/catenin复合物通过Wnt信号传导途径参与胃癌的发生发展 [24-25]。此外,CDH17表达上调可影响GSK3的活性,抑制β-catenin-AXIN-APC-GSK3复合物的形成,稳定β-catenin表达水平,可使其与转录因子LEF/TCF结合,诱导Cyclin D1的产生,从而促进细胞增殖,抑制细胞凋亡(图2) [26]。有研究认为β-半乳糖苷结合蛋白Galectin-3在胃癌侵袭过程中的异常表达对CDH17起调控作用,但具体调控机制有待进一步研究 [27-28]。
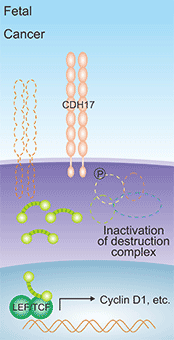
图1. CDH17诱导Cyclin D1的产生 [26]
此外,利用慢病毒介导的microRNA干扰技术发现,抑制CDH17表达后,MMP-2和MMP-9的活性明显降低,说明CDH17表达上调可增强MMP-2和MMP-9的活性,导致细胞外基质的降解和重塑,利于肿瘤细胞的转移 [17]。另有报道,CDH17通过整合Ras/Raf/MEK/ERK信号通路,在胃癌细胞的增殖和肿瘤的生长中发挥重要作用(图3) [29]。在肝癌中,敲低CDH17表达,导致Wnt/β-catenin信号通路失活,肿瘤生长受到抑制,因此推测靶向CDH17可灭活Wnt信号通路并激活抑癌基因,促进肿瘤细胞的凋亡 [30]。
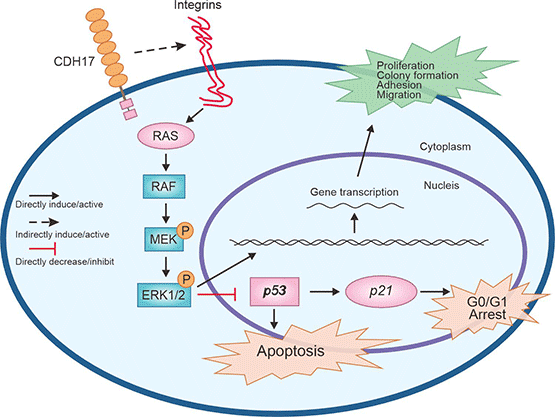
图3. CDH17介导Ras/Raf/MEK/ERK信号通路 [29]
4. CDH17在癌症治疗中的作用
CDH17作为新型钙黏蛋白,在细胞粘附、细胞识别、组织器官的发育和形态的维持等方面发挥重要作用。细胞间的粘附及运动能力的失调是肿瘤发生发展的重要机制之一。近年来有关于CDH17与肿瘤关系的靶向研究越来越多,主要见于胃癌、肝癌、结肠癌及胰腺癌等胃肠道恶性肿瘤。
4.1 CDH17和胃癌
CDH17最早被发现在胃癌中过度表达。临床研究中,对71例胃癌患者的癌组织和正常胃组织进行半定量PCR、免疫组化及Western blot分析发现CDH17的表达水平与胃癌的组织学类型、肿瘤侵袭和淋巴结转移均呈正相关。在CDH17过表达的BGC-823细胞株中,胃腺癌的增殖、侵袭及迁移能力增强。RNA干扰CDH17表达,发现下调CDH17能够抑制MKN-45胃癌细胞的增殖、粘附和侵袭能力,同时NF-κB信号转导通路被抑制、其下游蛋白(VEGF-C和MMP-9)减少 [31-33]。
4.2 CDH17和肝癌
研究表明CDH17与肝癌发生也有一定的关系。采用RT-PCR方法对57例肝癌组织及正常肝组织进行分析,结果显示CDH17在肝癌组织中的阳性表达率显著高于正常肝组织,说明CDH17与肝癌的发生有关,提示CDH17可能作为早期诊断肝癌的肿瘤标志物 [34]。对34例肝内胆管癌研究发现CDH17表达与肿瘤的分化程度和血管侵犯有关,敲除CDH17,发现CDH17表达下调,促进血管生成有关的MTF-1和胎盘生长因子PLGF的表达,进而诱导肿瘤血管生成 [11, 35]。另有研究发现CDH17的剪接异构体和基因多态性增加正常人群肝癌的发生风险 [26]。
4.3 CDH17和结肠癌
一项对45例结肠癌标本的研究发现,CDH17的表达下调与结肠癌的进展及淋巴结转移有显著关系。下调结肠癌细胞中CDH17基因的表达水平,可以抑制结肠癌细胞的侵袭和转移过程。对原发部位的结肠癌组织和其转移部位的癌组织中CDH17表达进行比较,发现二者之间的表达一致。因此,CDH17有望成为结肠癌转移检测的标志物 [22, 36]。
4.4 CDH17和食管癌
有研究分析食管癌组织中CDH17的表达情况与食管癌患者临床特征的关系,发现在分化良好的食管癌组织中有较高的CDH17免疫反应,而低分化的食管腺癌组织中CDH17的表达水平较低或表达不明显,证明食管腺癌的CDH17免疫反应与患者的临床特征具有一定的关系 [37]。在食管鳞状细胞癌ESCC中,CDH17 CpG 岛甲基化状态增强,CDH17表达水平降低,CDH17 CpG 岛的甲基化状态与CDH17的表达呈负相关 [38-39]。因此,CDH17可成为食管癌治疗的新靶点。
4.5 CDH17和胰腺癌
一项对胰腺导管癌患者CDH17表达情况的研究发现,高分化癌中CDH17表达高于低分化癌者,Kaplan-Meier曲线分析表明CDH17高表达与患者预后生存有关 [40]。低CDH17表达与肿瘤去分化相关,由于相关研究甚少,因而CDH17与胰腺癌临床病理特征及预后之间的关系还有待进一步探讨。
4.6 CDH17和其它癌症
对CDH17与卵巢上皮癌关系的研究发现,CDH17在低分化、高分期的肿瘤中高表达 [41]。单变量分析表明,CDH17高表达与预后不良有关。在导管内乳头状粘液性肿瘤(IPMN)中,CDH17诱导IPMN的肠型分化及癌变,随着IPMN级别的增高,CDH17与之呈正相关 [42]。总而言之,陆续的研究提示CDH17可能成为胃癌、肝癌和结直肠癌等恶性肿瘤诊断和预后的标志物,监测肿瘤的进展及复发。
5. CDH17的临床研究前景
目前已有多款基于CDH17的临床药物在研(表1),主要用于胃肠道肿瘤治疗。其中,3款为双特异性抗体:ARB-202(CDH17 x CD3),ARB-001.T(CDH17 x CD3),BI-905711(CDH17 x DR5/TRAIL-R2);1款CAR-T药物CHM-2101;2款单抗。晚期消化道恶性肿瘤的治疗,往往是以化疗为基础的单药或联合治疗方案,虽然化疗药物及联合治疗策略迭代更新,但疗效始终未能获得突破性进展。肿瘤靶向免疫治疗提供了全新高效、安全、低毒的治疗策略,有望改变目前困境。现有研究成果已表明,靶向干扰CDH17可以抑制肿瘤生长。因此,CDH17作为新发现的肝肠钙黏蛋白,正成为胃肠道肿瘤靶向治疗的热门靶点!
| 药物 | 靶点 | 作用机制 | 药物类型 | 在研适应症 | 在研机构 | 最高研发状态 |
|---|---|---|---|---|---|---|
| ARB-202 | CDH17;CD3 | 免疫调节剂; CDH17调节剂;CD3调节剂 |
双特异性抗体 | 胆管癌; 结直肠癌; 胃肠道肿瘤;胃癌;胆道肿瘤;胰腺癌;肝癌;胰腺腺泡癌 |
Arbele | 临床1期 |
| BI-905711 | DR5;CDH17 | DR5激动剂; CDH17调节剂 |
双特异性抗体 | 弥漫性大B细胞淋巴瘤; 胃癌; 胆管癌;胃肠道肿瘤;胰腺癌;食管癌;胆管癌 |
C.H. Boehringer Sohn AG & Co. KG;勃林格殷格翰(中国)投资有限公司 Boehringer Ingelheim (China) Investment Co., Ltd.;勃林格殷格翰 Boehringer Ingelheim GmbH |
临床1期 |
| Anti-CDH17 mAbs (ProAlt) | CDH17 | CDH17调节剂 | 单克隆抗体 | 转移性结直肠癌;转移性黑色素瘤 | 普罗克拉拉生物科学股份有限公司 Proclara Biosciences, Inc. |
临床前 |
| ARB-001.M | CDH17 | CDH17拮抗剂 | 双特异性抗体 | 胃肠道肿瘤 | Arbele | 临床前 |
| ARB-001.T | CD3;CDH17 | CDH17调节剂 | 双特异性抗体 | 结直肠癌;肝癌; 胃癌 |
Arbele | 临床前 |
| ARB-201 | CDH17 | CDH17拮抗剂;CD3抑制剂 | 双特异性抗体 | 结直肠癌 | Arbele | 临床前 |
| CHM-2101 | CDH17 | CDH17调节剂;基因转移 | CAR-T | 结直肠癌; 胃肠道肿瘤; 神经内分泌肿瘤 |
/ | 临床前 |
| CHM-2301 | CDH17 | CDH17调节剂 | CAR-NK | 实体瘤 | / | 临床前 |
| Anti-CDH17-based antibody drug conjugates(ProAlt) | CDH17 | CDH17调节剂 | ADC;单克隆抗体 | 结直肠癌 | Protein Alternatives SL | 药物发现 |
表1:CDH17的临床药物在研
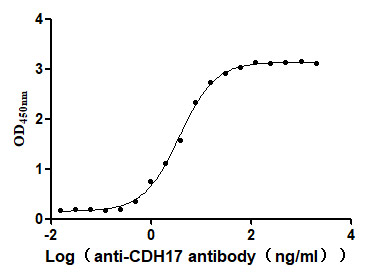
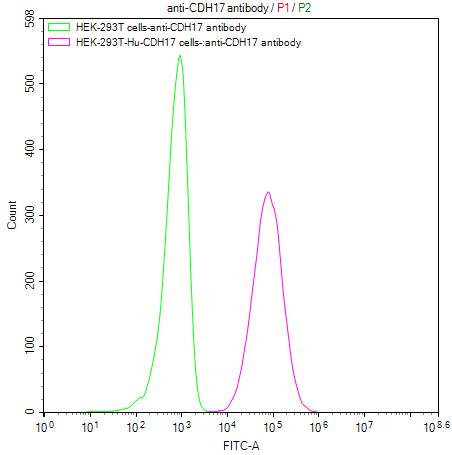
为鼎力协助各药企针对CDH17在胃肠道肿瘤等其它肿瘤在临床中的研究,CUSABIO推出CDH17活性蛋白产品,(Code:CSB-MP613267HU),助力您在CDH17机制方面的研究或其潜在临床价值的探索。
CDH17 Protein&Human CDH17 Stable Cell Line
参考文献:
[1] Hulpiau, Paco, Ismail Sahin Gul, and Frans Van Roy. "Evolution of cadherins and associated catenins. "The Cadherin Superfamily: Key Regulators of Animal Development and Physiology (2016): 13-37.
[2] Ma, Yi-Shih, et al. "Bisdemethoxycurcumin suppresses human osteosarcoma U-2 OS cell migration and invasion via affecting the PI3K/Akt/ NF-κB, PI3K/Akt/GSK3β and MAPK signaling pathways in vitro." Oncology Reports 48.6 (2022): 1-10.
[3] Yu, Weina, et al. "Cadherin signaling in cancer: its functions and role as a therapeutic target." Frontiers in oncology 9 (2019): 989.
[4] Türkeli, Ahmet, et al. "Anti-VEGF treatment suppresses remodeling factors and restores epithelial barrier function through the E -cadherin/β-catenin signaling axis in experimental asthma models." Experimental and Therapeutic Medicine 22.1 (2021): 1 -9.
[5] Ramesh, P. K. Immunolocalization of Gingival E-Cadherin Expression in Smokers and Non-Smokers with Chronic Periodontitis. diss. KSR Institute of Dental Science and Research, Tiruchengode, 2020.
[6] Harki, Olfa, et al. "Inhibition of Vascular Endothelial Cadherin Cleavage Prevents Elastic Fiber Alterations and Atherosclerosis Induced by Intermittent Hypoxia in the Mouse Aorta." International Journal of Molecular Sciences 23.13 (2022): 7012.
[7] Koziolek, Michael, et al. "Urine E-cadherin: a marker for early detection of kidney injury in diabetic patients." journal of clinical medicine 9.3 (2020 ): 639.
[8] Loh, Chin-Yap, et al. "The E-cadherin and N-cadherin switch in epithelial-to-mesenchymal transition: signaling, therapeutic implications, and challenges." Cells 8.10 (2019): 1118.
[9] Yu, Chong, et al. "The lncRNA ZNF667-AS1 Inhibits Propagation, Invasion, and Angiogenesis of Gastric Cancer by Silencing the Expression of N-Cadherin and VEGFA." Journal of Oncology 2022 (2022).
[10] Zhang, Jinyao, et al. "Clinical significance of ALDH1A1 expression and its association with E-cadherin and N-cadherin in resected large cell neuroendocrine carcinoma." Translational Oncology 19 (2022): 101379.
[11] Takamura, Masaaki, et al. "Involvement of liver-intestine cadherin in cancer progression." medical molecular morphology 46 (2013): 1-7.
[12] Ordóñez, Nelson G. "Cadherin 17 is a novel diagnostic marker for adenocarcinomas of the digestive system." Advances in anatomic pathology 21.2 (2014): 131-137.
[13] Gray, Michelle E., and Marcos Sotomayor. "Crystal structure of the nonclassical cadherin-17 N-terminus and implications for its adhesive binding mechanism." Acta Crystallographica Section F: Structural Biology Communications 77.3 (2021): 85-94.
[14] Caporuscio, Christian, et al. "Immunoaffinity enrichment LC-MS/MS quantitation of CDH17 in tissues." Bioanalysis 12.20 (2020): 1439- 1447.
[15] Horsfield, Julia, et al. "Cadherin-17 is required to maintain pronephric duct integrity during zebrafish development." Mechanisms of development 115.1-2 (2002): 15-26.
[16] Huang, Li-Ping, et al. "Up-regulation of cadherin 17 and down-regulation of homeodomain protein CDX2 correlate with tumor progression and unfavorable prognosis in epithelial ovarian cancer." International Journal of Gynecologic Cancer 22.7 (2012).
[17] Jiang, Xiao-jie, et al. "CDH17 alters MMP-2 expression via canonical NF-κB signalling in human gastric cancer." Gene 682 (2019): 92-100.
[18] Liu, Xinjian, et al. "Disruption of oncogenic liver-intestine cadherin (CDH17) drives apoptotic pancreatic cancer death." Cancer Letters 454 (2019): 204-214.
[19] Xia, Peng, et al. "Surface-Engineered Extracellular Vesicles with CDH17 Nanobodies to Efficiently Deliver Imaging Probes and Chemo -Photothermal Drugs for Gastric Cancer Theragnostic." Advanced Functional Materials (2022): 2209393.
[20] Xia, Peng, et al. "Surface-Engineered Extracellular Vesicles with CDH17 Nanobodies to Efficiently Deliver Imaging Probes and Chemo -Photothermal Drugs for Gastric Cancer Theragnostic." Advanced Functional Materials (2022): 2209393.
[21] Feng, Zijie, et al. "Potent suppression of neuroendocrine tumors and gastrointestinal cancers by CDH17CAR T cells without toxicity to normal tissues." Nature Cancer 3.5 (2022): 581-594.
[22] Pei, Xiao Meng, et al. "The diagnostic significance of CDH17-positive circulating tumor cells in patients with colorectal cancer." Expert Review of Molecular Diagnostics just-accepted (2023).
[23] Wu, Cunen, et al. "Interaction between Wnt/β-catenin pathway and microRNAs regulates epithelial-mesenchymal transition in gastric cancer." International journal of oncology 48.6 (2016): 2236-
[22] 6.
[24] Kaszak, Ilona, et al. "Role of cadherins in cancer-a review." international journal of molecular sciences 21.20 (2020): 7624.
[25] Wang, Qianwen, et al. "MICAL2 contributes to gastric cancer cell migration via Cdc42-dependent activation of E-cadherin/β-catenin signaling pathway ." Cell Communication and Signaling 20.1 (2022): 136.
[26] Lee, Nikki P., et al. "Role of cadherin-17 in oncogenesis and potential therapeutic implications in hepatocellular carcinoma. "Biochimica et Biophysica Acta (BBA)-Reviews on Cancer 1806.2 (2010): 138-145.
[27] Huang, Hsiang-Wei, et al. "Association between inflammation and function of cell adhesion molecules influence on gastrointestinal cancer development." Cells 10.1 (2021): 67.
[28] Maher, John, and David M. Davies. "CAR-Based Immunotherapy of Solid Tumours-A Survey of the Emerging Targets. "Cancers 15.4 (2023): 1171.
[29] Lin, Zhaohu, et al. "Targeting cadherin-17 inactivates Ras/Raf/MEK/ERK signaling and inhibits cell proliferation in gastric cancer." PLoS One 9.1 ( 2014): e85296.
[30] Wang, Yonggang, et al. "Anti-cadherin-17 antibody modulates beta-catenin signaling and tumorigenicity of hepatocellular carcinoma." ploS one 8.9 ( 2013): e72386.
[31] Motoshita, Junichi, et al. "Molecular characteristics of differentiated-type gastric carcinoma with distinct mucin phenotype: LI -cadherin is associated with intestinal phenotype." Pathology international 56.4 (2006): 200-205.
[32] Wang, Jin, et al. "Cadherin-17 induces tumorigenesis and lymphatic metastasis in gastric cancer through activation of NFκB signaling pathway." Cancer biology & therapy 14.3 (2013): 262-270.
[33] Qiu, Hai-bo, et al. "Targeting CDH17 suppresses tumor progression in gastric cancer by downregulating Wnt/β-catenin signaling." ploS one 8.3 (2013): e56959.
[34] Su, Min-Cheng, et al. "Cadherin-17 is a useful diagnostic marker for adenocarcinomas of the digestive system." Modern Pathology 21.11 (2008): 1379- 1386.
[35] Takamura, Masaaki, et al. "Loss of liver-intestine cadherin in human intrahepatic cholangiocarcinoma promotes angiogenesis by up-regulating metal- responsive transcription factor-1 and placental growth factor." International journal of oncology 36.1 (2010): 245-254.
[36] Harding, J. J., et al. "371P A phase Ia/b, open-label, multicentre study of the TRAILR2 agonist BI 905711 in patients (pts) with advanced gastrointestinal (GI) cancers." Annals of Oncology 33 (2022): S706.
[37] Panarelli, Nicole C., et al. "Tissue-specific cadherin CDH17 is a useful marker of gastrointestinal adenocarcinomas with higher sensitivity than CDX2 ." American journal of clinical pathology 138.2 (2012): 211-222.
[38] Shenoy, U. Sangeetha, et al. "Molecular implications of HOX genes targeting multiple signaling pathways in cancer." Cell biology and toxicology (2022) : 1-30. : 1-30.
[39] Ignatova, Ekaterina Olegovna, et al. "Clinical significance of molecular subtypes of gastrointestinal tract adenocarcinoma." World Journal of Gastrointestinal Oncology 14.3 (2022): 628.
[40] Liu, Xinjian, et al. "Disruption of oncogenic liver-intestine cadherin (CDH17) drives apoptotic pancreatic cancer death." Cancer Letters 454 (2019): 204-214.
[41] Huang, Li-Ping, et al. "Up-regulation of cadherin 17 and down-regulation of homeodomain protein CDX2 correlate with tumor progression and unfavorable prognosis in epithelial ovarian cancer." International Journal of Gynecologic Cancer 22.7 (2012).
[42] Karimi, S. S., T. Valyi-Nagy, and M. F. Gonzalez. "TTF-1 Immunoexpression in Primary Rectal Adenocarcinoma with Brain Metastasis. "American Journal of Clinical Pathology 156.Supplement_1 (2021): S63-S63.