Recombinant Mouse Endoplasmic reticulum chaperone BiP (Hspa5)
In Stock-
货号:CSB-YP010827MO
-
规格:¥2208
-
图片:
-
其他:
产品详情
-
纯度:Greater than 90% as determined by SDS-PAGE.
-
基因名:
-
Uniprot No.:
-
别名:Hspa5; Grp78; Endoplasmic reticulum chaperone BiP; EC 3.6.4.10; 78 kDa glucose-regulated protein; GRP-78; Binding-immunoglobulin protein; BiP; Heat shock protein 70 family protein 5; HSP70 family protein 5; Heat shock protein family A member 5; Immunoglobulin heavy chain-binding protein
-
种属:Mus musculus (Mouse)
-
蛋白长度:Full Length of Mature Protein
-
来源:Yeast
-
分子量:72.5kDa
-
表达区域:20-655aa
-
氨基酸序列EEEDKKEDVGTVVGIDLGTTYSCVGVFKNGRVEIIANDQGNRITPSYVAFTPEGERLIGDAAKNQLTSNPENTVFDAKRLIGRTWNDPSVQQDIKFLPFKVVEKKTKPYIQVDIGGGQTKTFAPEEISAMVLTKMKETAEAYLGKKVTHAVVTVPAYFNDAQRQATKDAGTIAGLNVMRIINEPTAAAIAYGLDKREGEKNILVFDLGGGTFDVSLLTIDNGVFEVVATNGDTHLGGEDFDQRVMEHFIKLYKKKTGKDVRKDNRAVQKLRREVEKAKRALSSQHQARIEIESFFEGEDFSETLTRAKFEELNMDLFRSTMKPVQKVLEDSDLKKSDIDEIVLVGGSTRIPKIQQLVKEFFNGKEPSRGINPDEAVAYGAAVQAGVLSGDQDTGDLVLLDVCPLTLGIETVGGVMTKLIPRNTVVPTKKSQIFSTASDNQPTVTIKVYEGERPLTKDNHLLGTFDLTGIPPAPRGVPQIEVTFEIDVNGILRVTAEDKGTGNKNKITITNDQNRLTPEEIERMVNDAEKFAEEDKKLKERIDTRNELESYAYSLKNQIGDKEKLGGKLSSEDKETMEKAVEEKIEWLESHQDADIEDFKAKKKELEEIVQPIISKLYGSGGPPPTGEEDTSEKDEL
Note: The complete sequence including tag sequence, target protein sequence and linker sequence could be provided upon request. -
蛋白标签:N-terminal 6xHis-tagged
-
产品提供形式:Liquid or Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
缓冲液:Tris-based buffer,50% glycerol
-
储存条件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保质期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
货期:3-7 business days
-
注意事项:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
产品描述:
The recombinant endoplasmic reticulum chaperone BiP (Hspa5) was produced as a fusion protein with an N-terminal 6xHis tag in the yeast cells. The encoding sequence contains 20-655aa of mouse Hspa5 protein. The purity of this full-length of mature Hspa5 protein reached up to 90% determined by SDS-PAGE. It migrated to a molecular mass band of 78-90 kDa on the gel. The slightly higher result is attributed to glycosylation. This recombinant Hspa5 protein can induce immune reactions in the immunized animals to thus get specific antibodies. Besides, it also may find uses in the tags and cell markers research area.
Heat shock protein family A member 5 (Hspa5), also known as GRP78 or Bip, is a member of heat shock proteins (HSPs) that are involved in proper protein folding, assembly of multiprotein complexes, as well as protein transport and degradation. In response to the accumulation of misfolded proteins in the ER, endoplasmic chaperone protein Hspa5 dissociates from ATF6-IRE1-PERK complex and then translocates into the lumen of the ER. This process unlocks the activity of the signal transducers previously blocked, which leads to the activation of the UPR signaling pathways. Recently, surface HSPA5 has been reported to be a potential receptor of some viruses, including the novel SARS-CoV-2.
-
Datasheet & COA:Please contact us to get it.
相关产品
靶点详情
-
功能:Endoplasmic reticulum chaperone that plays a key role in protein folding and quality control in the endoplasmic reticulum lumen. Involved in the correct folding of proteins and degradation of misfolded proteins via its interaction with DNAJC10/ERdj5, probably to facilitate the release of DNAJC10/ERdj5 from its substrate. Acts as a key repressor of the ERN1/IRE1-mediated unfolded protein response (UPR). In the unstressed endoplasmic reticulum, recruited by DNAJB9/ERdj4 to the luminal region of ERN1/IRE1, leading to disrupt the dimerization of ERN1/IRE1, thereby inactivating ERN1/IRE1. Accumulation of misfolded protein in the endoplasmic reticulum causes release of HSPA5/BiP from ERN1/IRE1, allowing homodimerization and subsequent activation of ERN1/IRE1. Plays an auxiliary role in post-translational transport of small presecretory proteins across endoplasmic reticulum (ER). May function as an allosteric modulator for SEC61 channel-forming translocon complex, likely cooperating with SEC62 to enable the productive insertion of these precursors into SEC61 channel. Appears to specifically regulate translocation of precursors having inhibitory residues in their mature region that weaken channel gating. May also play a role in apoptosis and cell proliferation.
-
基因功能参考文献:
- GRP78 regulates insulin resistance and macrophage polarization with selective IL-6 secretion in diet-induced obesity. PMID: 29242277
- These results uncover a novel role of GRP78 in reducing prion pathogenesis, suggesting that modulating its levels/activity may offer a novel opportunity for designing therapeutic approaches for these diseases. PMID: 28333162
- Endoplasmic reticulum stress leads to de novo biosynthesis of non-trimethylated GRP78, whereas homeostatic, METTL21A-dependent lysine 585-trimethylated GRP78 is reduced. PMID: 28912266
- GRP78 haploinsufficiency for up to 2 years of age has no major deleterious effect in rodents of different genetic background. PMID: 28145503
- GRP78 promotes cigarette smoke-induced inflammatory response and mucus hyperproduction in airway epithelial cells, likely through upregulation of necroptosis and subsequent activation of NF-kappaB and AP-1 pathways. PMID: 29445274
- siRNA of Grp78 disturbed the fusion of mouse palate cultured in vitro, suggesting a role in the palate development during embryogenesis.. PMID: 29233047
- prolonged endoplasmic reticulum stress promotes apoptosis via a p53-dependent inhibition of BiP expression PMID: 28622297
- role in acinar-to-ductal metaplasia and pancreatic ductal adenocarcinoma PMID: 28461470
- analysis of the effects of triptolide on cell proliferation, cell cycle and the expression of GRP78 in nasopharyngeal carcinoma PMID: 27391061
- candidate genes that modulate Hspa5 expression in the retina, were examined. PMID: 27881906
- These results indicate that GRP78, but not nutritional status, is a potent up-regulator of hepatic PTC-mRNA levels during induction of ER stress in vivo. PMID: 28383761
- Data suggest that activation of GRP78/Ire1/Xbp1 pathway of ER stress-unfolded protein response is involved in mouse decidualization. PMID: 27283502
- These results demonstrate a key role for GRP78 in alveolar epithelial cell survival. PMID: 26816051
- These results indicate that GRP78, an endoplasmic reticulum chaperon of the HSP70 family, is a novel host factor involved at multiple steps of the Japanese encephalitis virus life cycle and could be a potential therapeutic target. PMID: 28053106
- Genetic or pharmacologic inhibition of the HSPA5-GPX4 pathway enhanced gemcitabine sensitivity by disinhibiting ferroptosis in vitro and in both subcutaneous and orthotopic animal models of PDAC. PMID: 28130223
- Endoplasmic reticulum stress gene GRP78 is involved in signaling pathway during hepatitis B virus-mediated hepatocarcinogenesis. PMID: 26567840
- data show that Med inhibits ER stress-induced apoptosis and promotes osteoblast cell survival by targeting GRP78. PMID: 27316719
- These results suggested the important roles of endoplasmic reticulum-related chaperons, Bip and SIL1, in Alzheimer's disease-like tau hyperphosphorylation. PMID: 25575678
- We show that chronic VPA treatment did not modify the ATXN3 inclusion load and astrogliosis in affected brain regions However, VPA chronic treatment was able to increase GRP78 protein levels at 30 weeks of age, one of its known neuroprotective effects PMID: 26505994
- these findings reveal a novel critical role of GRP78 in regulating ER stress-mediated apoptosis in cartilage development and the molecular mechanisms involved. PMID: 26370957
- ISM is a novel vascular permeability inducer that functions through cell-surface GRP78-mediated Src activation as well as induction of apoptosis PMID: 25952901
- BIP increased surface CD19 molecule expression intensity and IL-10(+), PD-L1(hi) and FasL(hi) B cells induced by BIP share the CD19(hi) phenotype PMID: 26655428
- These studies demonstrate that BiP is critical for myelinating cell survival and contributes to the protective response of oligodendrocyte against inflammatory demyelination. PMID: 26631473
- These studies imply GRP78, but not GRP94, is required for mammary gland development. PMID: 24953136
- A highly specific monoclonal antibody against GRP78 suppressed AKT activation and increased apoptosis in the cPten(f/f) tumors. PMID: 25684138
- AdGRP78 reduced expression of lipogenic genes and plasma triglycerides in the db/db strain. Both G5 and G8 protein levels increased as did total biliary cholesterol PMID: 26365598
- Heterozygous mutant BiP mice revealed motor disabilities in aging. PMID: 25405877
- findings underscore the specific and prominent role of SKIP and GRP78 in the regulation of insulin-dependent PI 3-kinase signaling in skeletal muscle PMID: 26376412
- STZ injection i.p. rapidly induced up-regulation of the ER stress marker, the prosurvival chaperone glucose-regulated protein 78. With the development of diabetes, the expression of GRP78 decreases. PMID: 25529350
- Downregulation of GRP78 abolishes the activity of exogenous Syn, indicating that it is the primary target of Syn. PMID: 25124556
- high glucose induced astrocytic activation in both in vivo and in vitro diabetic models, in which modulation of GRP78 would be an important event in this activation PMID: 24056253
- This study showed that that inflammation leads to citrullination of GRP78 in beta-cells; opening new avenues for biomarker development and therapeutic intervention. PMID: 25204978
- Systemic gene transfer of binding immunoglobulin protein (BiP) prevents disease progression in murine collagen-induced arthritis. PMID: 25228326
- Data suggest that the balance between Cripto and Grp78 expression levels might be crucial in cancer development and may account for the increased tumorigenesis in Cripto heterozygous mice. PMID: 24805056
- Deficiency of the BiP cochaperone ERdj4 causes constitutive endoplasmic reticulum stress and metabolic defects. PMID: 24336520
- GRP78 might play an important role during the process of mouse embryo implantation, and GRP78 expression was mainly regulated by active blastocysts and maternal oestrogen. PMID: 24242779
- there is a causal link between a humoral response to GRP78 and the progression of cancer in a murine melanoma model PMID: 21164368
- GRP78 is a novel regulator for PTEN-loss-mediated liver injury and cancer progression. PMID: 24141775
- Matrin 3 interacts specifically with the heat shock proteins glucose-regulated protein 78, GRP75 and glutathione S-transferase pi isoform 2. PMID: 24491357
- Reg2 induces glucose-regulated peptide 78 (GRP78) expression via the Akt-mTORC1 axis. PMID: 24801175
- downregulated miR-181b induces neuroprotection against ischemic injury through negatively regulating HSPA5 and UCHL1 protein levels PMID: 23900885
- the interaction of amelogenin with Grp78/Bip contributed to cell proliferation, rather than correlate with the osteogenic differentiation PMID: 24167599
- IRE1alpha dissociates from BiP and inhibits ER stress-mediated apoptosis in the process of cartilage development. PMID: 23816533
- Data indicate that GRP78 is expressed in metastatic mammary tumors. PMID: 23470966
- Data suggest that transgenic mice that overproduce GRP78 in pancreatic beta cells are leaner than non-transgenic littermates and are protected against development of glucose intolerance, insulin resistance, and endoplasmic reticulum stress in islets. PMID: 23475366
- Knockdown of GRP78/BiP increased the accumulation of AR aggregates and significantly higher levels of caspase-3 activity and cell apoptosis. PMID: 23618905
- BiP was particularly observed in the pathological vasculature and retinal microvascular endothelial cells, and the increase of BiP expression was correlated with retinal neovascularization. PMID: 23544152
- These findings demonstrate a sensitive requirement for the endoplasmic reticulum chaperone BiP/GRP78 during axon outgrowth and pathfinding in the developing mammalian brain. PMID: 22821687
- GRP78 is required for adipocyte differentiation, glucose homeostasis, and balanced secretion of adipokines. PMID: 23180827
- NPGPx is essential for releasing excessive ER stress by enhancing GRP78 chaperone activity to maintain physiological homeostasis. PMID: 23123197
显示更多
收起更多
-
亚细胞定位:Endoplasmic reticulum lumen. Melanosome. Cytoplasm. Cell surface.
-
蛋白家族:Heat shock protein 70 family
-
数据库链接:
KEGG: mmu:14828
STRING: 10090.ENSMUSP00000028222
UniGene: Mm.330160
Most popular with customers
-
Recombinant Human Tumor necrosis factor receptor superfamily member 14 (TNFRSF14), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
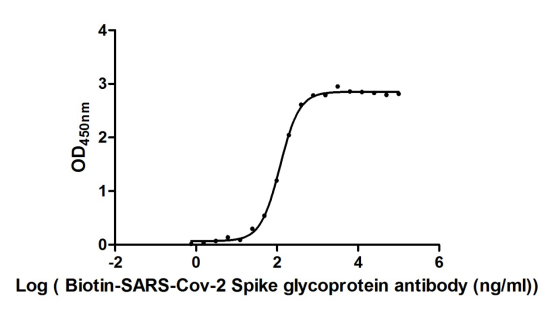
Recombinant Severe acute respiratory syndrome coronavirus 2 Spike glycoprotein (S), partial (Active)
Express system: Mammalian cell
Species: Severe acute respiratory syndrome coronavirus 2 (2019-nCoV) (SARS-CoV-2)
-
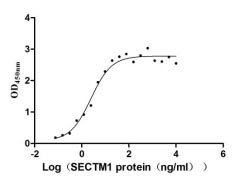
Recombinant Human Secreted and transmembrane protein 1 (SECTM1), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
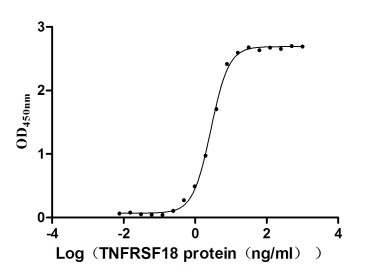
Recombinant Human Tumor necrosis factor receptor superfamily member 18 (TNFRSF18), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
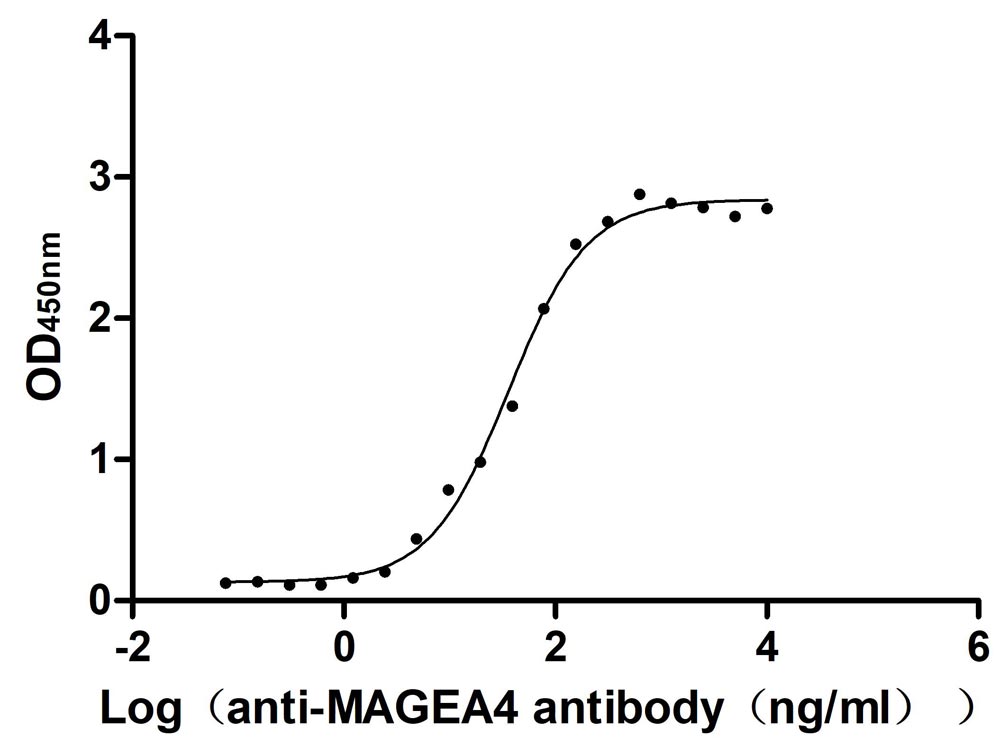
Recombinant Human Melanoma-associated antigen 4 (MAGEA4) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Rat Intestinal-type alkaline phosphatase 1 (Alpi) (Active)
Express system: Mammalian cell
Species: Rattus norvegicus (Rat)
-
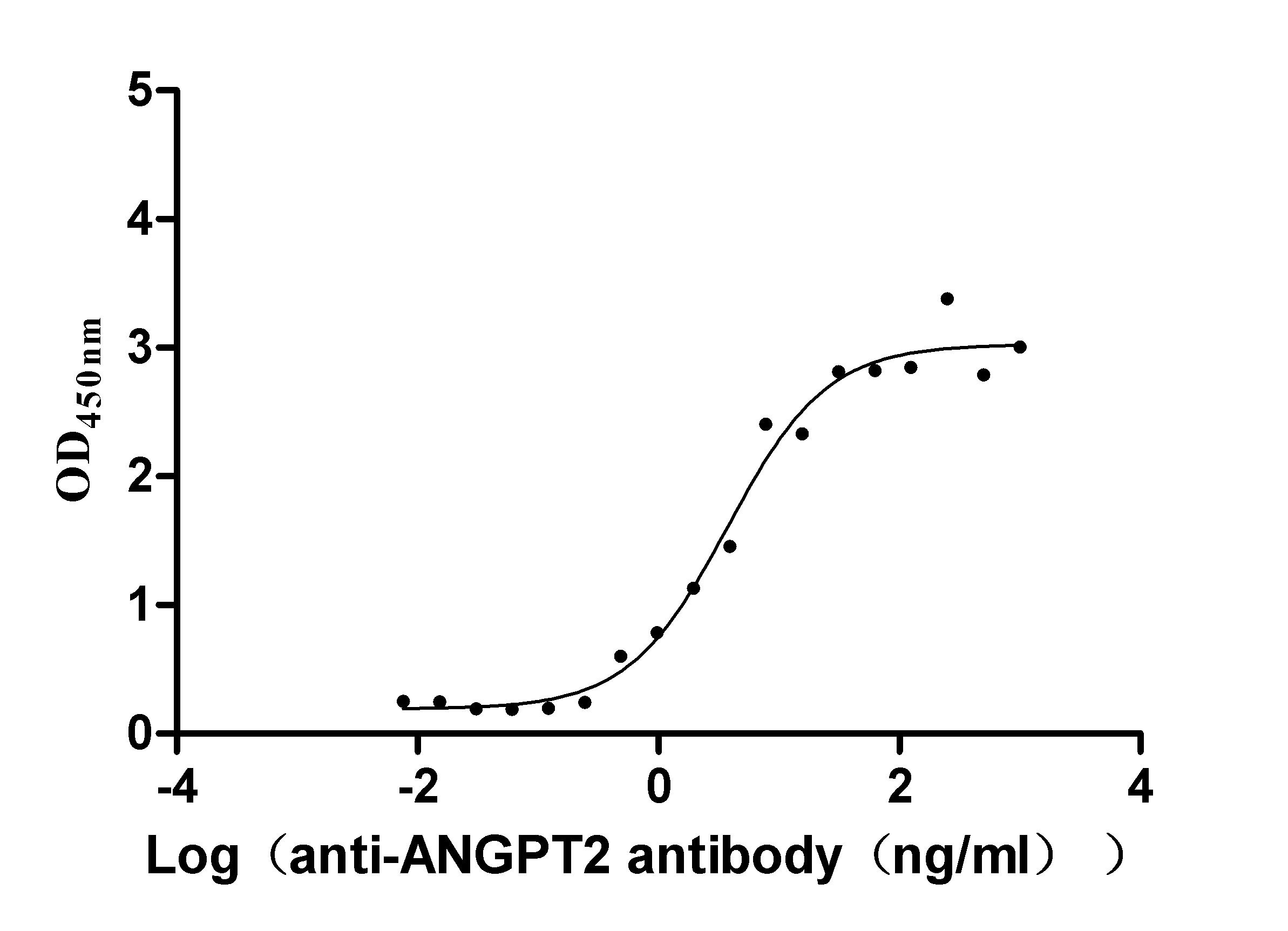
Recombinant Dog Angiopoietin-2 (ANGPT2) (Active)
Express system: Mammalian cell
Species: Canis lupus familiaris (Dog) (Canis familiaris)
-
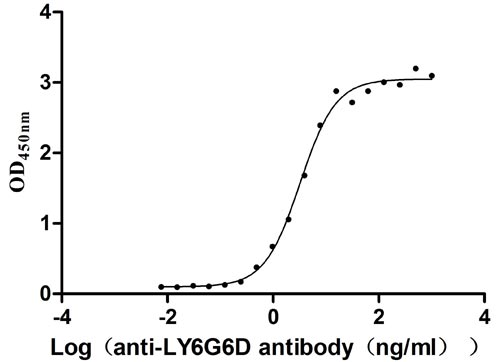
Recombinant Human Lymphocyte antigen 6 complex locus protein G6d (LY6G6D) (Active)
Express system: Yeast
Species: Homo sapiens (Human)