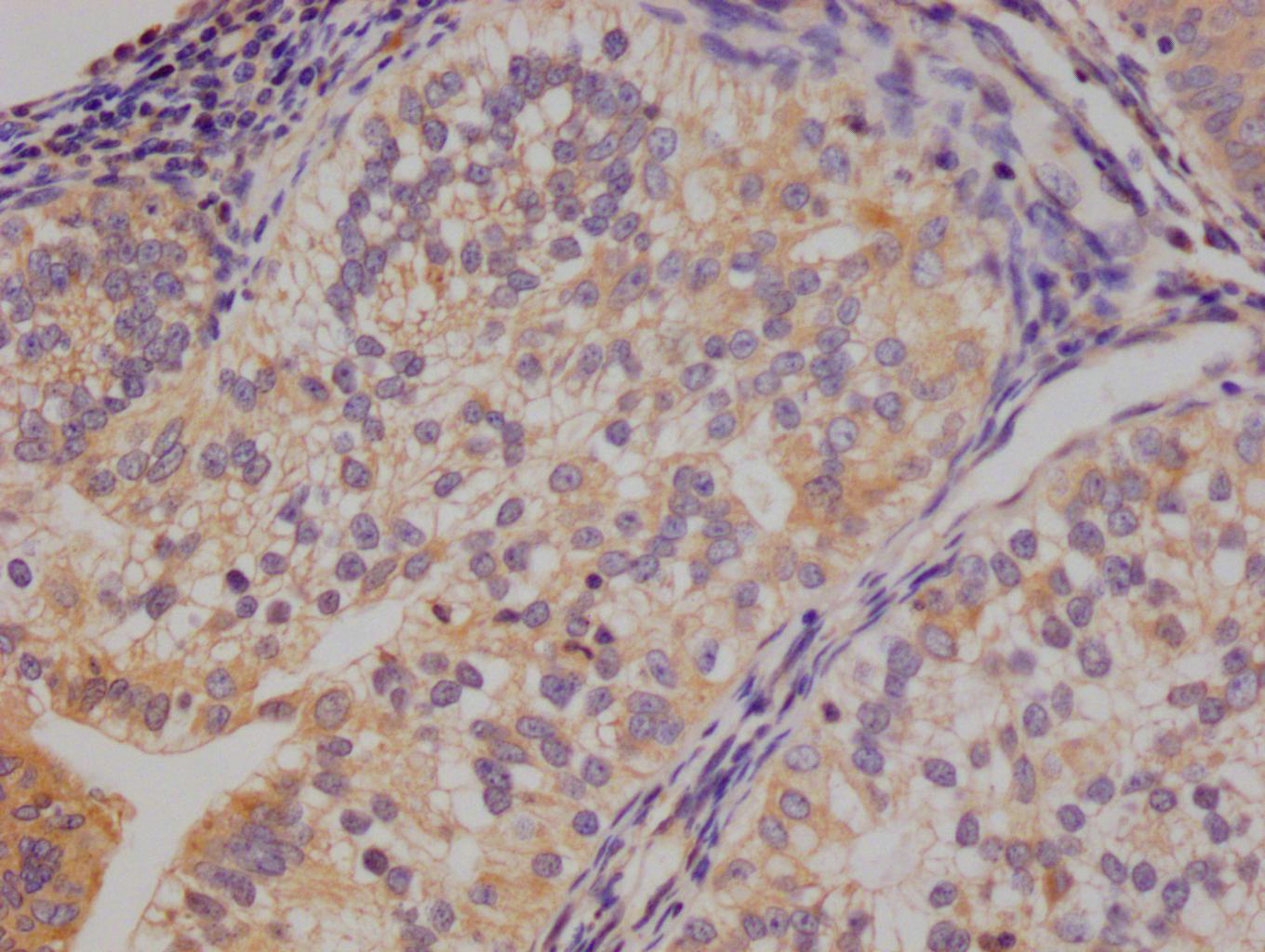
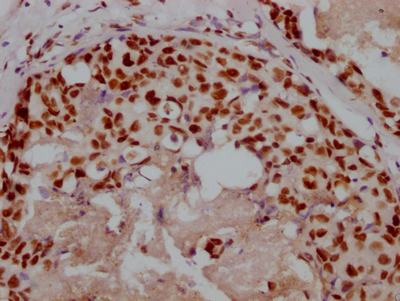
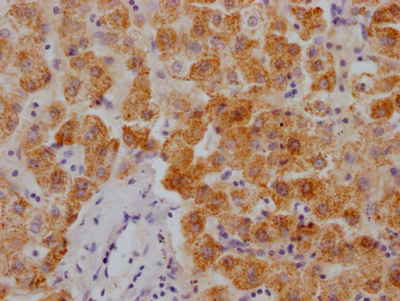
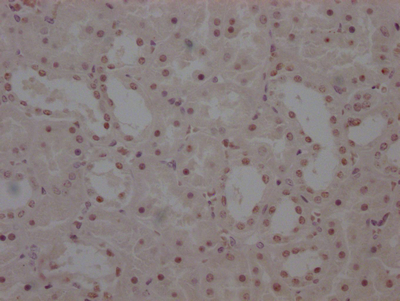
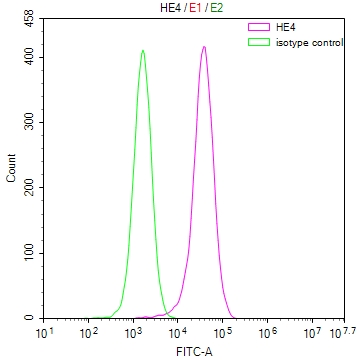
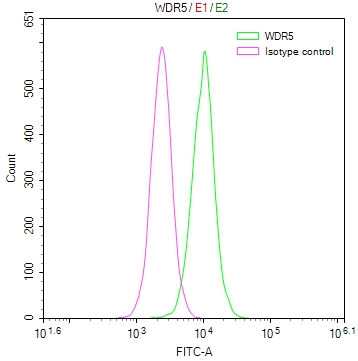
Phospho-RAD52 (Y104) Antibody
-
货号:CSB-PA040147
-
规格:¥880
-
其他:
产品详情
-
Uniprot No.:P43351
-
基因名:RAD52
-
别名:DNA repair protein RAD52 antibody; DNA repair protein RAD52 antibody; DNA repair protein RAD52 homolog antibody; RAD 52 antibody; Rad52 antibody; RAD52 homolog (S. cerevisiae) antibody; RAD52 homolog (S. cerevisiae) antibody; RAD52 homolog antibody; RAD52_HUMAN antibody; Recombination protein RAD52 antibody; Recombination protein RAD52 antibody; Rhabdomyosarcoma antigen MU RMS 40.23 antibody
-
宿主:Rabbit
-
反应种属:Human,Mouse,Monkey
-
免疫原:Synthesized peptide derived from Human Rad52 around the phosphorylation site of Y104.
-
免疫原种属:Homo sapiens (Human)
-
标记方式:Non-conjugated
-
抗体亚型:IgG
-
纯化方式:The antibody was affinity-purified from rabbit antiserum by affinity-chromatography using epitope-specific immunogen.
-
浓度:It differs from different batches. Please contact us to confirm it.
-
保存缓冲液:Liquid in PBS containing 50% glycerol, 0.5% BSA and 0.02% sodium azide.
-
产品提供形式:Liquid
-
应用范围:WB, IHC, ELISA
-
推荐稀释比:
Application Recommended Dilution WB 1:500-1:2000 IHC 1:100-1:300 ELISA 1:20000 -
Protocols:
-
储存条件:Upon receipt, store at -20°C or -80°C. Avoid repeated freeze.
-
货期:Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time maybe differs from different purchasing way or location, please kindly consult your local distributors for specific delivery time.
相关产品
靶点详情
-
功能:Involved in double-stranded break repair. Plays a central role in genetic recombination and DNA repair by promoting the annealing of complementary single-stranded DNA and by stimulation of the RAD51 recombinase.
-
基因功能参考文献:
- Rad52 competes with Ku for binding to S-region double-strand DNA breaks (DSB) free ends, where it facilitates a DSB synaptic process, which favours intra-S region recombination. PMID: 28176781
- Due to its similarity to RAD52, we hypothesized that RDM1 potentially repairs DNA doublestrand breaks arising through DNA replication. PMID: 29845285
- The mechanism by which RAD52 depletion causes synthetic lethality in BRCA1 mutant cancer cells depends on the 5' endonuclease EEPD1, which normally functions to cleave stressed replication forks to initiate HR repair. PMID: 29145865
- inhibition of ataxia telangiectasia mutated (ATM) protein by siRNA or inhibitor treatment demonstrated that the acetylation of RAD52 at DSB sites is dependent on the ATM protein kinase activity, through the formation of RAD52, p300/CBP, SIRT2, and SIRT3 foci at DSB sites PMID: 29590107
- n a cohort of patients with typical symptoms of ischemic heart disease, a common single nucleotide polymorphism of the human RAD52 gene has a role in increased hazard of death, showing that it may influence aging PMID: 29024686
- RAD52 gene polymorphism is associated with colorectal cancer. PMID: 26735576
- our study demonstrated that RAD52 polymorphisms were associated with colorectal cancer in a Chinese Han cohort PMID: 29245274
- DNA-bound RAD52 is efficient at capturing ssDNA in trans. PMID: 28329678
- Structure of the human DNA-repair protein RAD52 containing surface mutations has been reported. PMID: 27487923
- Rad52 inverse strand exchange plays an important role in RNA-templated double strand break repair in vivo. PMID: 28602639
- The mitotic DNA synthesis is RAD52 dependent, and RAD52 is required for the timely recruitment of MUS81 and POLD3 to common fragile sites in early mitosis. PMID: 27984745
- Human RAD52-null cells retain a significant level of single-strand annealing (SSA) activity demonstrating perforce that additional SSA-like activities must exist in human cells. Moreover, the SSA activity associated with RAD52 is involved in, but not absolutely required for, most homology-directed repair (HDR) subpathways. Specifically, a deficiency in RAD52 impaired the repair of DNA DSBs. PMID: 28549257
- The C-terminal region of yRad52, but not of hRAD52, is involved in ssDNA annealing. This suggests that the second DNA binding site is required for the efficient ssDNA annealing by yRad52. We propose an updated model of Rad52-mediated ssDNA annealing. PMID: 27362509
- Study discovered two cis-expression quantitative trait loci SNPs in the RAD52 gene that are associated with its expression and are also associated with lung squamous cell carcinomas (LUSC) risk. PMID: 26013599
- these results implicate increased RAD52 expression in both genetic susceptibility and tumorigenesis of upper aerodigestive tract and lung squamous cell carcinoma tumors. PMID: 25793373
- RAD52 rs7963551 single nucleotide polymorphism was significantly associated with glioma risk. PMID: 25012956
- This study found that only the RAD52 rs7963551 single nucleotide polymorphism was significantly associated with hepatitis B virus related - hepatocellular carcinoma risk. PMID: 24729511
- Our findings reveal a novel RAD52/MUS81-dependent mechanism that promotes cell viability and genome integrity in checkpoint-deficient cells, and disclose the involvement of MUS81 to multiple processes after replication stress PMID: 24204313
- Our data did not show association between LIG4 and RAD52 SNPs and SLE, its clinical manifestations or ethnicity in the tested population. PMID: 24415301
- RAD52 mutation is associated with leukemia. PMID: 23836560
- RAD52 is an alternative repair pathway of RAD51-mediated homologous recombination, and a target for therapy in cells deficient in the BRCA1-PALB2-BRCA2 repair pathway. PMID: 22964643
- Single nucleotide polymorphisms in RAD52 are associated with myelodysplastic syndromes. PMID: 23339595
- Both RAD52 variants and protein expression can predict platinum resistance, and RAD52 variants appeared to predict prognosis in cervical cancer patients. PMID: 23209746
- rs7963551 located at the hsa-let-7 binding site may alter expression of RAD52 and contribute to the development of breast cancer in Chinese women. PMID: 23188672
- The recruitment kinetics of Rad52 is slower than that of Mdc1, but exhibits the same dependence on LET PMID: 22860035
- Silencing of the Rad52 gene in fractionated group of A549 cells made the cells radiosensitive. PMID: 22001234
- RAD52(Y104pCMF) specifically targets and wraps ssDNA. Phosphorylation at Y104 enhances ssDNA annealing activity of RAD52 by attenuating dsDNA binding. PMID: 21804533
- Rad52 can respond to DNA double-strand breaks and replication stalling independently of BRCA2 PMID: 21148102
- the possibility of sumoylation playing an important role in the nuclear transport of RAD52 PMID: 20190268
- This study demonstrated a positive correlation between molecular beacon fluorescence intensity, RAD52 gene expression and both gamma ionising radiation and antineoplastic concentration in human TK6 cells. PMID: 19799994
- hRad52 stably binds and wraps both, protein free and replication protein A-coated ssDNA. PMID: 20081207
- differential effects of Rad52p overexpression on gene targeting and extrachromosomal homologous recombination in a human tumor cell line PMID: 11809887
- Analysis of the human replication protein A:Rad52 complex: evidence for crosstalk between RPA32, RPA70, Rad52 and DNA PMID: 12139939
- Crystal structure of the homologous-pairing domain from the human Rad52 recombinase in the undecameric form. PMID: 12191481
- crystal structure of the single-strand annealing domain PMID: 12370410
- RAD52 may play a role in transcription regulation and in targeting DNA damage on transcription active loci to recombinational repair PMID: 12372413
- women with Ser346ter nonsense polymorphism of RAD52 not at increased risk of breast cancer in case-control study PMID: 12376524
- coordinated WRN and RAD52 activities are involved in replication fork rescue after DNA damage PMID: 12750383
- Rad52 facilitates homologous recognition between single-stranded DNA and duplex-DNA through a process that involves unwinding or transient unpairing of the interacting duplex via a novel three-stranded intermediate that does not lead to strand exchange. PMID: 14690434
- the ternary complex of hRad52 and XPF/ERCC1 is the active species that processes recombination intermediates generated during the repair of DNA double strand breaks and in homology-dependent gene targeting events PMID: 14734547
- For both yeast Rad52 and HsRad52, the yield of strand-exchange reactions was proportional to the fractional A.T content of the DNA substrates, but both enzymes catalyzed exchange with substrates that contained up to at least 50% G.C PMID: 15205482
- formation of a stoichiometric complex between HsRad52 and single-stranded DNA was found to be critical for strand exchange activity PMID: 15205484
- analysis of residues important for DNA binding in the full-length human Rad52 protein PMID: 15571718
- RAD52 Y415X polymorphism is not associated with epithelial ovarian cancer in Australian women PMID: 15670896
- Interestingly, presence of hRad52 restores the ability of hRad51 binding to such DNA targets as well. PMID: 16018971
- Rad52 protein functions by binding to single-stranded DNA formed as intermediates of recombination rather than by binding to the unprocessed DNA double-strand break. PMID: 17040915
- Data show that DNA repair synthesis, catalyzed by human DNA polymerase eta (poleta) acting upon the priming strand of a D loop, leads to capture and annealing of the second end of a resected DSB in reactions mediated by RAD52 protein. PMID: 18313388
- Rad52 aligns two recombining DNA molecules within the first and second DNA binding sites to stimulate the homology search and strand invasion processes. PMID: 18593704
- model for hRad52-mediated DNA annealing where ssDNA release and dsDNA zippering are coordinated through successive rearrangement of overlapping nucleoprotein complexes PMID: 19074292
- Of the 21 loci screened, RAD52 2259 and RAD52 GLN221GLU may be of importance to disease process and may be associated with papillary thyroid cancer risk in Saudi Arabian population. PMID: 19092295
显示更多
收起更多
-
亚细胞定位:Nucleus.
-
蛋白家族:RAD52 family
-
数据库链接:
HGNC: 9824
OMIM: 600392
KEGG: hsa:5893
STRING: 9606.ENSP00000351284
UniGene: Hs.410355
Most popular with customers
-
-
Phospho-YAP1 (S127) Recombinant Monoclonal Antibody
Applications: ELISA, WB, IHC
Species Reactivity: Human
-
-
-
-
-
-