ErbB signaling pathway
What Is ErbB?
ErbB (erythroblastic oncogene B) belongs to the epidermal growth factor receptor family and consists of an extracellular domain, a transmembrane domain, and a cytoplasmic tyrosine kinase domain. In humans, the ErbB family includes four members: ErbB1 (Her1), ErbB2 (Her2), ErbB3 (Her3), ErbB4 (Her4).
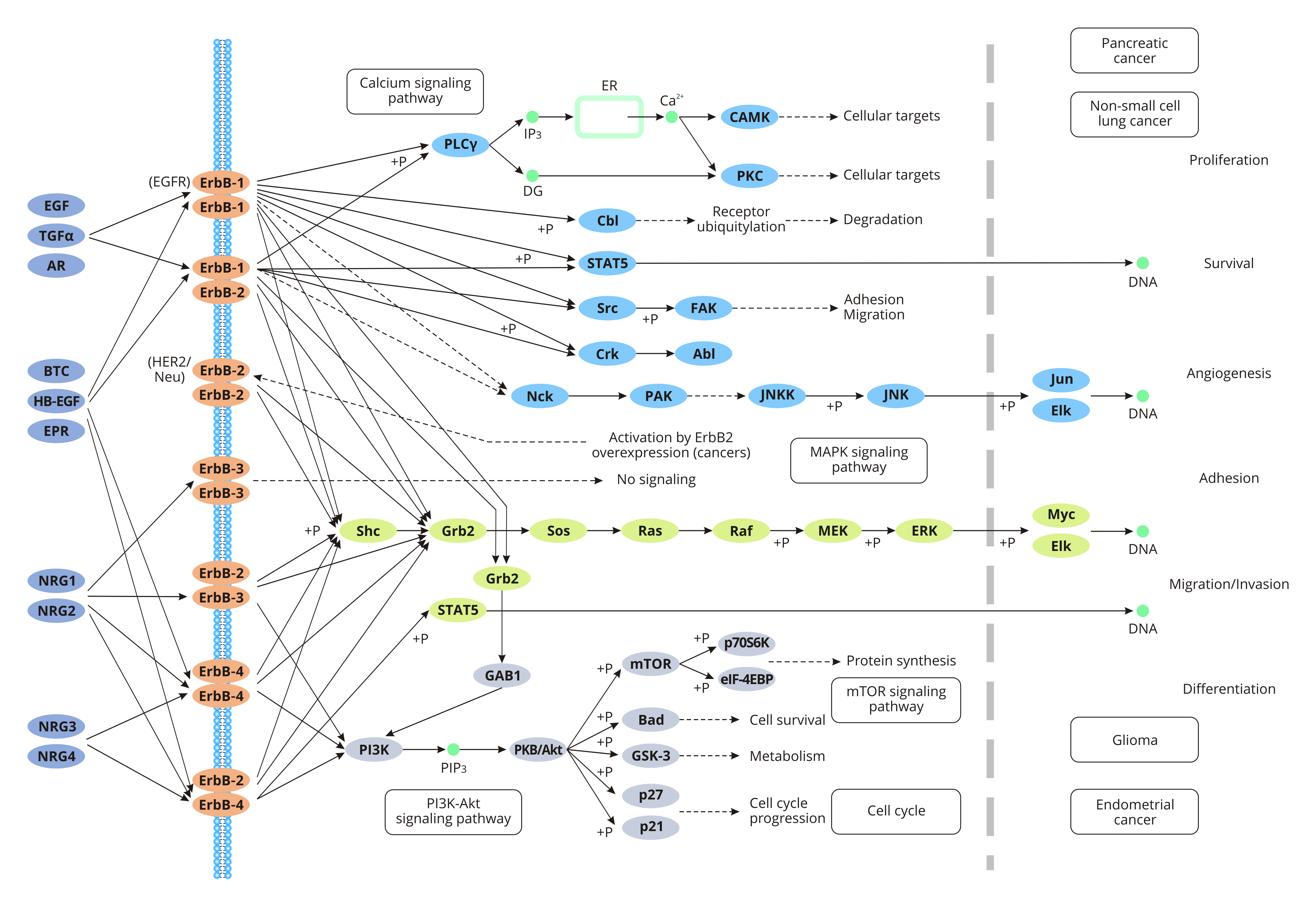
ErbB1 is also called EGFR (epidermal growth factor receptor). Normally, EGFR is expressed on the surface of epithelial cells. But it is often overexpressed in some tumor cells. The overexpression of EGFR is associated with metastasis, invasion and poor prognosis of tumor cells. EGFR is activated by binding to EGF or TGF-α (transforming growth factor α). Activated EGFR is converted from an inactive monomer to an active homodimer.
ErbB2 does not have a known ligand. But ErbB2 is the best candidate for the formation of a heterodimer with the other three receptors. Studies have revealed that amplification or overexpression of ErbB2 is associated with the development and progression of some breast cancers. ErbB2 has recently become an important biomaker and therapeutic target of some certain breast cancers.
ErbB3 is often seen in the nervous system, skin, urinary tract, and reproductive system of normal adult human. can bind to neuregulin 1 (NRG1) or NRG2. Due to lack of the kinase domain, ErbB3 needs to bind to the other three family members form an active heterodimer. And ErbB3 prefers the binding with ErbB2.
ErbB4 has many ligands such as NRG1, NRG2, NRG3, NRG4, epiregulin, HB-EGF, and betacellulin. ErbB4 mutation is detected in many cancers.
What Is ErbB Signaling Pathway?
The ErbB signaling pathway refers to multiple processes by which ErbB members dimerize or heterodimerize through the binding with numerous signal transducers to promote the autophosphorylation and subsequent downstream signaling cascades.
The Function of ErbB Signaling Pathway
The ErbB signaling regulates cell proliferation, migration, differentiation, apoptosis, and cell motility by mediating the PI3K/Akt pathway, the JAK/STAT pathway, and the MAPK signaling pathway.
ErbB family members and some of their ligands are often overexpressed, amplified, or mutated in many forms of cancer, making them important therapeutic targets.
The Process of ErbB Signaling Pathway
Among the four ErbB receptors, ErbB1 and ErbB4 are popular to be studied.
Upon binding to EGF or TGF-α, EGFR is dimerized to a homodimer with activity. EGFR dimerization stimulates its intracellular protein-tyrosine kinase activity, which phosphorylates the Tyr residues. These phosphorylated residues provide docking sites for the protein signaling molecules with an SH2 (Src homology 2) or a PTB domain. Protein interactions in activated receptor complexes stimulate ras proteins, leading to the development of a phosphorylation cascade and activation of mitogen-activated protein kinase (MAPK), thereby activating phosphatidylinositol kinase-3 (PI3K) -Akt, MPAK, and JNK signaling pathways. These signaling pathways further trigger gene transcription, which promotes DNA synthesis and cell proliferation.
NRG1 has a high affinity with ErbB4. NRG1 directly binds to ErbB3 or ErbB4, which induces homologous or allogenic dimerization of the latter. The dimerization activates tyrosine kinase activity. Once the ErbB receptors are activated, ErbB2 binds to ErbB4 to facilitate tyrosine autophosphorylation. This process subsequently triggers the occurrence of downstream phosphorylation cascades and related signaling pathways. Signals transmitted by ErbB induce a wide range of biological functions, including cardiac development, synaptic formation, and proliferation and differentiation of Schwann cells. Studies have shown that NRG1 is involved in the development &differentiation of oligodendrocytes and the proliferation of Schwann cells through the P13K-Akt pathway.