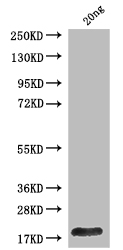
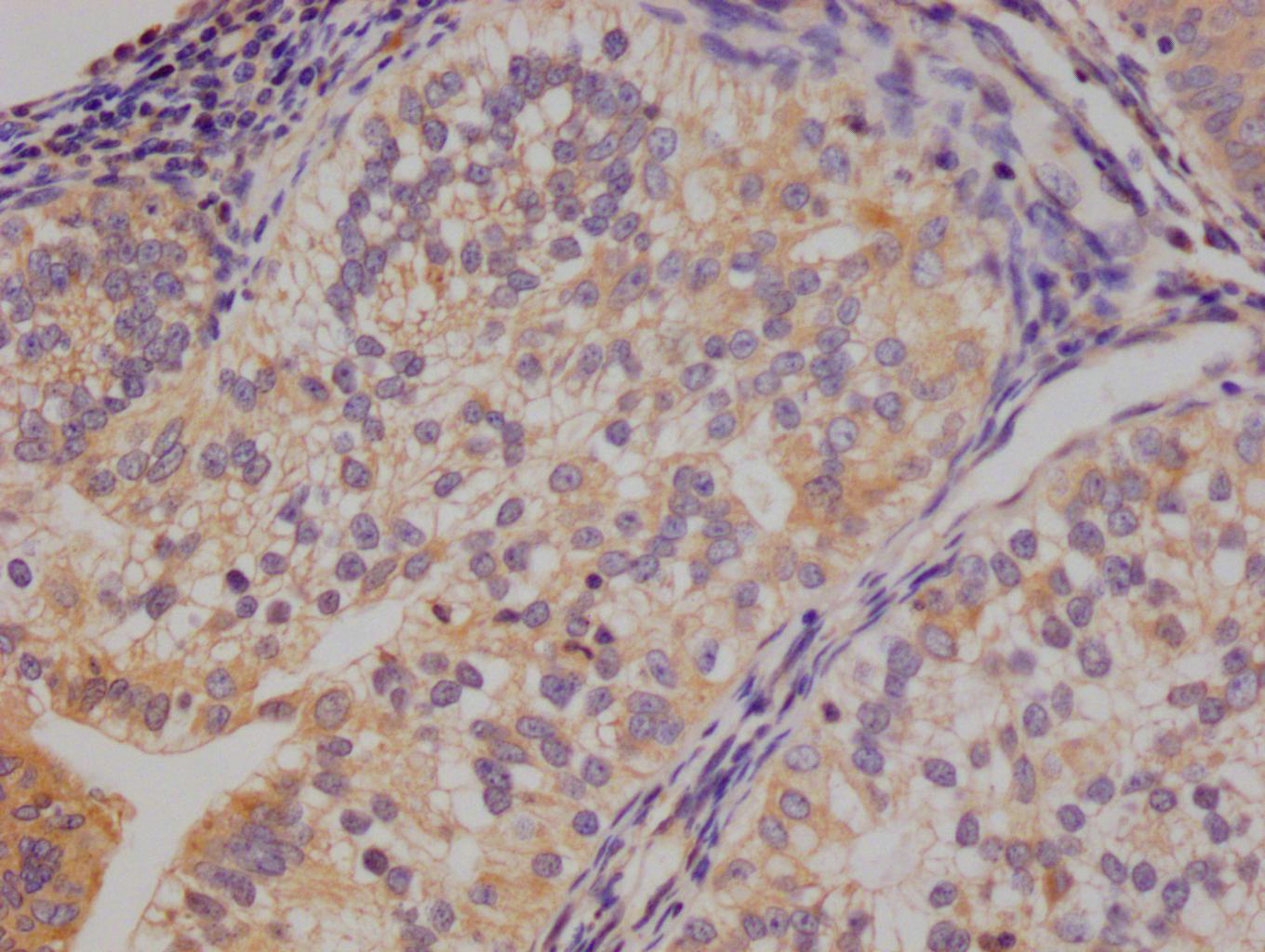
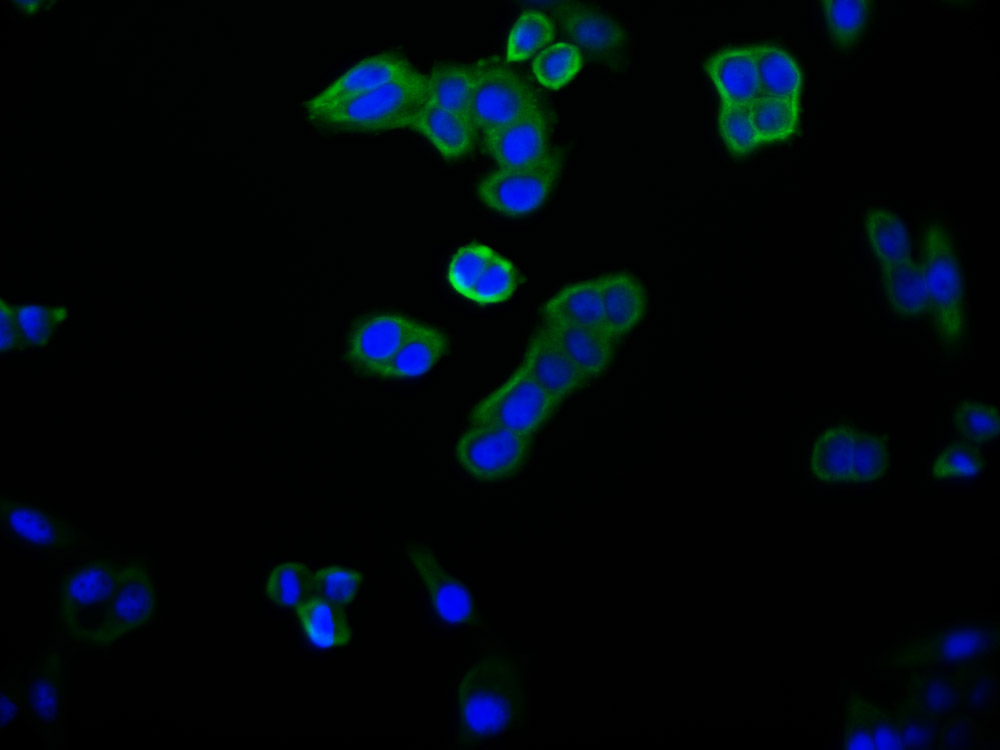
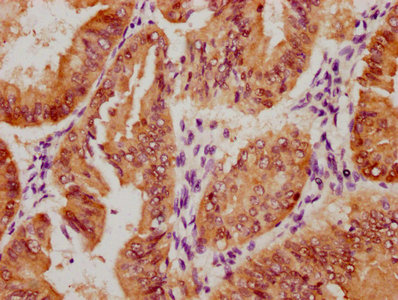
lacY Antibody
-
货号:CSB-PA365806XA01ENV
-
规格:¥440
-
促销:
-
图片:
-
其他:
产品详情
-
Uniprot No.:P02920
-
基因名:lacY
-
别名:Lactose permease (Lactose-proton symport) lacY b0343 JW0334
-
反应种属:Escherichia coli (strain K12)
-
免疫原:Recombinant Escherichia coli (strain K12) lacY protein
-
免疫原种属:Escherichia coli (strain K12)
-
标记方式:Non-conjugated
-
克隆类型:Polyclonal
-
抗体亚型:IgG
-
纯化方式:Protein G
-
浓度:It differs from different batches. Please contact us to confirm it.
-
保存缓冲液:Preservative: 0.03% Proclin 300
Constituents: 50% Glycerol, 0.01M PBS, pH 7.4 -
产品提供形式:Liquid
-
应用范围:ELISA, WB
-
Protocols:
-
储存条件:Upon receipt, store at -20°C or -80°C. Avoid repeated freeze.
-
货期:Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time maybe differs from different purchasing way or location, please kindly consult your local distributors for specific delivery time.
相关产品
靶点详情
-
功能:Responsible for transport of beta-galactosides into the cell, with the concomitant import of a proton (symport system). Can transport lactose, melibiose, lactulose or the analog methyl-1-thio-beta,D-galactopyranoside (TMG), but not sucrose or fructose. The substrate specificity is directed toward the galactopyranosyl moiety of the substrate.
-
基因功能参考文献:
- Binding kinetics of alpha-galactopyranoside homologs with fluorescent aglycones of different sizes or shapes were determined with Escherichia coli LacY by FRET from Trp151 in the LacY binding site to the fluorophores. LacY specificity is directed toward the galactopyranoside ring. The pathway for the substrate entering from the periplasmic side is wider than the calculated pore diameters in periplasmic open X-ray struc... PMID: 29602806
- Glu325 exhibits a pKa of 10.5 +/- 0.1 that is insensitive to the presence of galactopyranoside. Protonation of Glu325 specifically is required for effective sugar binding to LacY PMID: 28154138
- Data indicate that the nanobodies (Nbs) bind stoichiometrically with nanomolar affinity to the periplasmic face of lactose permease (LacY) primarily to the C-terminal six-helix bundle. PMID: 27791182
- Molecular dynamics simulations indicated that the deprotonation of Glu325 induced the opening of the periplasmics side and partial closure of the cytoplasmic side of LacY, while protonation of the Glu269 caused a stable cross-domain salt-bridge (Glu130-Arg344) and completely closed the cytoplasmic side. PMID: 27090495
- Double-replacement mutants of conserved Gly-Gly pairs bind galactoside with affinities 10-20-fold higher than that of the pseudo-WT or WT LacY. Moreover, site-directed alkylation of a periplasmic Cys replacement indicates that the periplasmic cavity becomes readily accessible in the double-replacement mutants. PMID: 27438891
- WT LacY in complex with the great majority of the Nbs exhibits varied increases in access of sugar to the binding site with an increase in association rate constants (kon) of up to approximately 50-fold (reaching 10(7) M(-1) s(-1)). PMID: 25512549
- The lactose permease gene (lacY) was overexpressed in the septuple knockout mutant of Escherichia coli.. inactivate the lactose repressor, induce the lactose operon, and as a result stimulate overall lactose consumption and conversion. PMID: 23725289
- Data indicate that opening of the periplasmic cavity not only limits access of sugar to the binding site of lactose permease (LacY) but also controls the rate of closing of the cytoplasmic cavity. PMID: 24872451
- Exploit chemical denaturation to determine the unfolding free energy of LacY and employ Trp residues as site-specific thermodynamic probes. Trp LacY mutants are created with the individual Trps situated at mirror image positions on the two LacY domains. PMID: 24530957
- findings are consistent with the interpretation that the electrogenic reaction induced by sugar binding is due to rearrangement of charged residues in LacY and that this reaction is blocked by mutation of each member of the Tyr236/Glu269/His322 triad PMID: 24152072
- Trp replacements for tightly interacting Gly-Gly pairs in LacY stabilize an outward-facing conformation. PMID: 23671103
- Proper fatty acid composition rather than an ionizable lipid amine is required for full transport function of lactose permease from Escherichia coli PMID: 23322771
- Data indicate that transmembrane domains (TMs) orientation for lactose permease LacY as a function of membrane lipid composition. PMID: 22969082
- study led to the discovery that LacY activity is a major physiological source of expression costs in the lac operon PMID: 22605776
- analysis of the intermediate conformational state of LacY PMID: 22355148
- Results indicate that LacY exhibits specificity directed toward the galactopyranosyl moiety of the glycoside to be transported. PMID: 22106930
- LacY is highly dynamic, and binding of a galactopyranoside causes closing of the inward-facing cavity with opening of a complementary outward-facing cavity. PMID: 21995338
- analysis of the interaction between helices V and I and its role in the transport mechanism of LacY protein PMID: 21730060
- Data show that MTS-gal is bound covalently, forming a disulfide bond with MTS-gal is bound covalently, forming a disulfide bond with Cys122 LacY and positioned between R144 and W151. PMID: 21593407
- topology of both CscB & PheP permeases is dependent on PE. However, CscB topology is governed by thermodynamic balance between opposing lipid-dependent electrostatic and hydrophobic interactions. PMID: 21454589
- Results describe a structural model of LacY generated by swapping the conformations of inverted-topology repeats identified in its two domains. PMID: 21315728
- Ser53, Gln60, and Phe354 are determined to be important in sugar transport during the periplasmic-open stage of the sugar transport cycle and the sugar is found to undergo an orientational change in order to escape the protein lumen. PMID: 20875429
- LacY exhibits uphill transport and native conformation of periplasmic domain P7 when expressed in a mutant in which phosphatidylcholine completely replaces phosphatidylethanolamine PMID: 20696931
- Electrogenic reactions accompanying downhill lactose/H(+) symport catalyzed by the lactose permease of Escherichia coli (LacY) have been assessed using solid-supported membrane-based electrophysiology with improved time resolution. PMID: 20568736
- demonstrate that sugar binding induces virtually the same global conformational change in LacY whether the protein is in the native bacterial membrane or is solubilized and purified in detergent. PMID: 20457922
- Data report the insertion of E. coli lactose permease in supported lipid bilayers of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol (POPG), in biomimetic molar proportions. PMID: 20096263
- Together with results from previous mutagenesis and cross-linking studies, these findings lead to a model in which replacement of Asp68 blocks a conformational transition involving helices II and IV that is important for opening the periplasmic cavity. PMID: 20043916
- analysis of sugar recognition by the LacY lactose permease of Escherichia coli PMID: 15364943
- LacY activity is dependent on subtle interactions between the helices, and mutations that disrupt interactions between helix IV and loop 6-7 or between helices II and IV also rescue transport in the cysteine154glycine mutant. PMID: 15909981
- reactivity of single-Cys mutants in helices I, III, VI, and XI of lactose permease with N-ethylmaleimide or methanethiosulfonate ethylsulfonate was studied in membrane vesicles. Most Cys replacements react with membrane-permeant alkylating agent NEM. PMID: 16566592
- Functional analyses of mutants in the homologous key residues provide strong evidence that they play a similar critical role in the mechanisms of CscB and LacY. PMID: 16574149
- structurally diverse lipids endow the membrane with similar properties necessary for the proper organization of protein domains in LacY that are highly sensitive to lipids as topological determinants PMID: 16698795
- thermodynamic analysis of ligand-induced conformational flexibility in the lactose permease of Escherichia coli PMID: 17003033
- model in which the single sugar-binding site in the approximate middle of the molecule is alternately exposed to either side of the membrane due to opening and closing of cytoplasmic and periplasmic hydrophilic cavities PMID: 17172438
- analysis of the x-ray structure of wild-type lactose permease (LacY) from Escherichia coli determined by manipulating phospholipid content during crystallization PMID: 17881559
- Double electron-electron resonance in conjunction with molecular modeling based on the x-ray structure provide strong support for the alternative access model and reveal a structure for the outward-facing conformer of LacY. PMID: 17925435
- The results provide direct support for the argument that transport via LacY involves opening and closing of a large periplasmic cavity. PMID: 18319336
- The results are consistent with the conclusion that LacY is protonated before sugar binding during lactose/H(+) symport in either direction across the membrane. PMID: 18567672
- ProP activity increased as LacY activity decreased when osmotic stress (increasing osmolality) was imposed on right-side-out cytoplasmic membrane vesicles. PMID: 18620422
- LacY involves at least 2 electrogenic reactions:a minor electrogenic step and a major electrogenic step. PMID: 19383792
- The data indicate that residues Ile40 and Asn245 play a primary role in gating the periplasmic cavity and provide further support for the alternating-access model. PMID: 19781551
显示更多
收起更多
-
亚细胞定位:Cell inner membrane; Multi-pass membrane protein.
-
蛋白家族:Major facilitator superfamily, Oligosaccharide:H(+) symporter (OHS) (TC 2.A.1.5) family
-
数据库链接:
KEGG: ecj:JW0334
STRING: 316385.ECDH10B_1356
Most popular with customers
-
-
YWHAB Recombinant Monoclonal Antibody
Applications: ELISA, WB, IF, FC
Species Reactivity: Human, Mouse, Rat
-
Phospho-YAP1 (S127) Recombinant Monoclonal Antibody
Applications: ELISA, WB, IHC
Species Reactivity: Human
-
-
-
-
-