gag-pol Antibody
-
货号:CSB-PA361030XA11MHK
-
规格:¥440
-
促销:
-
图片:
-
其他:
产品详情
-
Uniprot No.:P03355
-
基因名:gag-pol
-
别名:Gag-Pol polyprotein (Pr180gag-pol) [Cleaved into: Matrix protein p15 (MA), RNA-binding phosphoprotein p12 (pp12), Capsid protein p30 (CA), Nucleocapsid protein p10-Pol (NC-pol), Protease (PR) (EC 3.4.23.-) (p14), Reverse transcriptase/ribonuclease H (RT) (EC 2.7.7.49) (EC 2.7.7.7) (EC 3.1.26.4) (p80), Integrase (IN) (EC 2.7.7.-) (EC 3.1.-.-) (p46)], gag-pol
-
反应种属:Moloney murine leukemia virus
-
免疫原:Recombinant Moloney murine leukemia virus gag-pol protein (1444-1738aa)
-
免疫原种属:Moloney murine leukemia virus (isolate Shinnick) (MoMLV)
-
标记方式:Non-conjugated
-
克隆类型:Polyclonal
-
抗体亚型:IgG
-
纯化方式:Affinity-chromatography
-
浓度:It differs from different batches. Please contact us to confirm it.
-
保存缓冲液:Preservative: 0.03% Proclin 300
Constituents: 50% Glycerol, 0.01M PBS, pH 7.4 -
产品提供形式:Liquid
-
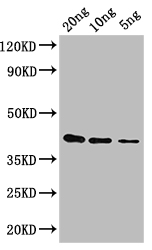
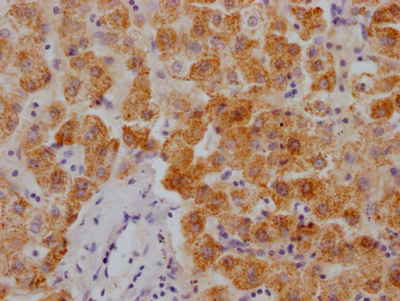
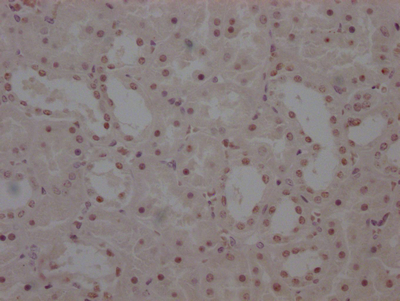
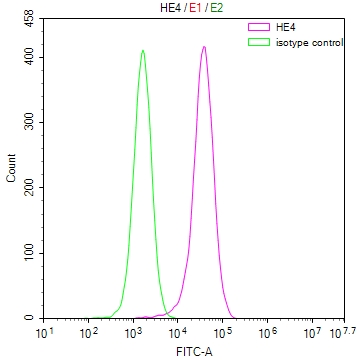
应用范围:ELISA, WB
-
Protocols:
-
储存条件:Upon receipt, store at -20°C or -80°C. Avoid repeated freeze.
-
货期:Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time maybe differs from different purchasing way or location, please kindly consult your local distributors for specific delivery time.
相关产品
靶点详情
-
功能:Plays a role in budding and is processed by the viral protease during virion maturation outside the cell. During budding, it recruits, in a PPXY-dependent or independent manner, Nedd4-like ubiquitin ligases that conjugate ubiquitin molecules to Gag-Pol, or to Gag-Pol binding host factors. Interaction with HECT ubiquitin ligases probably links the viral protein to the host ESCRT pathway and facilitates release.; Targets Gag and gag-pol polyproteins to the plasma membrane via a multipartite membrane binding signal, that includes its myristoylated N-terminus. Also mediates nuclear localization of the pre-integration complex.; Constituent of the pre-integration complex (PIC) which tethers the latter to mitotic chromosomes. This allows the integration of the viral genome into the host DNA.; Forms the spherical core of the virion that encapsulates the genomic RNA-nucleocapsid complex.; Involved in the packaging and encapsidation of two copies of the genome. Binds with high affinity to conserved UCUG elements within the packaging signal, located near the 5'-end of the genome. This binding is dependent on genome dimerization. Acts as a nucleic acid chaperone which is involved in rearrangement of nucleic acid secondary structures during gRNA retrotranscription.; The aspartyl protease mediates proteolytic cleavages of Gag and Gag-Pol polyproteins during or shortly after the release of the virion from the plasma membrane. Cleavages take place as an ordered, step-wise cascade to yield mature proteins. This process is called maturation. Displays maximal activity during the budding process just prior to particle release from the cell (Potential). Cleaves the translation initiation factor eIF4G leading to the inhibition of host cap-dependent translation.; RT is a multifunctional enzyme that converts the viral dimeric RNA genome into dsDNA in the cytoplasm, shortly after virus entry into the cell. This enzyme displays a DNA polymerase activity that can copy either DNA or RNA templates, and a ribonuclease H (RNase H) activity that cleaves the RNA strand of RNA-DNA heteroduplexes in a partially processive 3' to 5' endonucleasic mode. Conversion of viral genomic RNA into dsDNA requires many steps. A tRNA binds to the primer-binding site (PBS) situated at the 5' end of the viral RNA. RT uses the 3' end of the tRNA primer to perform a short round of RNA-dependent minus-strand DNA synthesis. The reading proceeds through the U5 region and ends after the repeated (R) region which is present at both ends of viral RNA. The portion of the RNA-DNA heteroduplex is digested by the RNase H, resulting in a ssDNA product attached to the tRNA primer. This ssDNA/tRNA hybridizes with the identical R region situated at the 3' end of viral RNA. This template exchange, known as minus-strand DNA strong stop transfer, can be either intra- or intermolecular. RT uses the 3' end of this newly synthesized short ssDNA to perform the RNA-dependent minus-strand DNA synthesis of the whole template. RNase H digests the RNA template except for a polypurine tract (PPT) situated at the 5' end of the genome. It is not clear if both polymerase and RNase H activities are simultaneous. RNase H probably can proceed both in a polymerase-dependent (RNA cut into small fragments by the same RT performing DNA synthesis) and a polymerase-independent mode (cleavage of remaining RNA fragments by free RTs). Secondly, RT performs DNA-directed plus-strand DNA synthesis using the PPT that has not been removed by RNase H as primers. PPT and tRNA primers are then removed by RNase H. The 3' and 5' ssDNA PBS regions hybridize to form a circular dsDNA intermediate. Strand displacement synthesis by RT to the PBS and PPT ends produces a blunt ended, linear dsDNA copy of the viral genome that includes long terminal repeats (LTRs) at both ends.; Catalyzes viral DNA integration into the host chromosome, by performing a series of DNA cutting and joining reactions. This enzyme activity takes place after virion entry into a cell and reverse transcription of the RNA genome in dsDNA. The first step in the integration process is 3' processing. This step requires a complex comprising the viral genome, matrix protein and integrase. This complex is called the pre-integration complex (PIC). The integrase protein removes 2 nucleotides from each 3' end of the viral DNA, leaving recessed CA OH's at the 3' ends. In the second step that requires cell division, the PIC enters cell nucleus. In the third step, termed strand transfer, the integrase protein joins the previously processed 3' ends to the 5' ends of strands of target cellular DNA at the site of integration. The last step is viral DNA integration into host chromosome.
-
基因功能参考文献:
- Findings indicate that reverse transcriptase is a relatively passive enzyme, able to polymerize on structured templates by exploiting their thermal breathing. PMID: 29165701
- X-ray crystal structure of the N-terminal region of Moloney murine leukemia virus integrase and its implications for viral DNA recognition has been reported. PMID: 28066922
- transmembrane protein associates with the assembling core particle through the R-peptide before budding and that this is the mechanism by which the budding virus acquires the envelope proteins. [TM R-peptide] PMID: 16690922
- analysis of how the murine leukaemia virus Env opens for membrane fusion PMID: 18800055
-
亚细胞定位:[Gag-Pol polyprotein]: Virion. Host cell membrane; Lipid-anchor. Host late endosome membrane; Lipid-anchor. Host endosome, host multivesicular body.; [Matrix protein p15]: Virion.; [Capsid protein p30]: Virion.; [Nucleocapsid protein p10-Pol]: Virion.; [Protease]: Virion.; [RNA-binding phosphoprotein p12]: Host cytoplasm.
-
数据库链接:
KEGG: vg:2193424
Most popular with customers
-
-
-
-
-
-
-
-
VDAC1 Recombinant Monoclonal Antibody
Applications: ELISA, WB, IHC
Species Reactivity: Human, Mouse, Rat