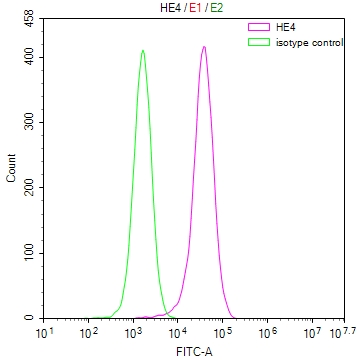
gag-pol Antibody
产品详情
-
产品名称:Rabbit anti-Human immunodeficiency virus type 1 group M subtype B gag-pol Polyclonal antibody
-
Uniprot No.:P04585
-
基因名:gag-pol
-
别名:gag-pol antibody; Gag-Pol polyprotein antibody; Pr160Gag-Pol) [Cleaved into: Matrix protein p17 antibody; MA); Capsid protein p24 antibody; CA); Spacer peptide 1 antibody; SP1 antibody; p2); Nucleocapsid protein p7 antibody; NC); Transframe peptide antibody; TF); p6-pol antibody; p6*); Protease antibody; EC 3.4.23.16 antibody; PR antibody; Retropepsin); Reverse transcriptase/ribonuclease H antibody; EC 2.7.7.49 antibody; EC 2.7.7.7 antibody; EC 3.1.26.13 antibody; Exoribonuclease H antibody; EC 3.1.13.2 antibody; p66 RT); p51 RT; p15; Integrase antibody; IN antibody; EC 2.7.7.- antibody; EC 3.1.-.-)] antibody
-
宿主:Rabbit
-
反应种属:Human immunodeficiency virus type 1 group M subtype B
-
免疫原:Recombinant Human immunodeficiency virus type 1 group M subtype B Gag-Pol polyprotein protein (1-440AA)
-
免疫原种属:Human immunodeficiency virus type 1 group M subtype B
-
标记方式:Non-conjugated
本页面中的产品,gag-pol Antibody (CSB-PA15387A0Rb),的标记方式是Non-conjugated。对于gag-pol Antibody,我们还提供其他标记。见下表:
-
克隆类型:Polyclonal
-
抗体亚型:IgG
-
纯化方式:>95%, Protein G purified
-
浓度:It differs from different batches. Please contact us to confirm it.
-
保存缓冲液:Preservative: 0.03% Proclin 300
Constituents: 50% Glycerol, 0.01M PBS, PH 7.4 -
产品提供形式:Liquid
-
应用范围:ELISA
-
Protocols:
-
储存条件:Upon receipt, store at -20°C or -80°C. Avoid repeated freeze.
-
货期:Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time maybe differs from different purchasing way or location, please kindly consult your local distributors for specific delivery time.
相关产品
靶点详情
-
功能:Mediates, with Gag polyprotein, the essential events in virion assembly, including binding the plasma membrane, making the protein-protein interactions necessary to create spherical particles, recruiting the viral Env proteins, and packaging the genomic RNA via direct interactions with the RNA packaging sequence (Psi). Gag-Pol polyprotein may regulate its own translation, by the binding genomic RNA in the 5'-UTR. At low concentration, the polyprotein would promote translation, whereas at high concentration, the polyprotein would encapsidate genomic RNA and then shut off translatio...显示更多
-
基因功能参考文献:
- cryoelectron microscopy of HIV-1 reverse transcriptase initiation complex PMID: 29695867
- Data show that the sequences preferred by HIV-1 RNase H are distributed in the HIV genome in a way suggesting selection for efficient RNA cleavage during replication. PMID: 29126318
- we studied for the first time the genetic diversity of HIV-1 BF recombinants and their evolution over time through in-depth phylogenetic analysis and multiple recombination detection methods involving 337 HIV-1 nucleotide sequences (25 near full-length (NFL) and 312 partial pol gene) obtained from Argentinean MSM PMID: 29244833
- These data imply that p53 can excise incorrect sugar in addition to base mispairs, thereby expanding the role of p53 in the repair of nucleic acids replication errors by HIV-1 reverse transcriptase. PMID: 28081035
- Drug resistance-related mutations T369V/I in the connection subdomain of HIV-1 reverse transcriptase severely impair viral fitness. PMID: 28279801
- Data suggest that the beta1'-beta2' motif of the RNase H domain may be responsible for displacing the connection domain from the polymerase cleft of putative monomeric p66; the unstable elongated p66 molecule may then readily dimerize with p51 to assume a stable dimeric conformation. PMID: 28627879
- Allosteric HIV-1 integrase inhibitors promote aberrant protein multimerization by directly mediating inter-subunit interactions PMID: 27503276
- Use of Capillary Electrophoresis to Study the Binding Interaction of Aptamers with Wild-Type, K103N, and Double Mutant (K103N/Y181C) HIV-1 RT : Studying the Binding Interaction of Wild-Type, K103N, and Double Mutant (K103N/Y181C) HIV-1 RT with Aptamers by Performing the Capillary Electrophoresis PMID: 27900665
- HIV-1 Reverse Transcriptase Polymerase and RNase H (Ribonuclease H) Active Sites Work Simultaneously and Independently. PMID: 27777303
- Rate-limiting Pyrophosphate Release by HIV Reverse Transcriptase Improves Fidelity PMID: 27777304
- Data indicate that inhibition of HIV-2 reverse transcriptases (RTs) by the nucleoside (NRTIs) tested showed a very similar trend in comparison to that of HIV-1 RT with lower Mg2+ concentrations. PMID: 27936595
- It has been found that the functional assembly of integrase through its fusion form with reverse transcriptase is critical for integrase to exert its nonenzymatic function. PMID: 27795445
- The amino acids at positions 76-100 of gp41 are required for it to bind to integrase. PMID: 27681265
- These results reveal an unexpected biological role ofHIV-1 Integrase binding to the viral RNA genome during virion morphogenesis and elucidate the mode of action of allosteric integrase inhibitors . PMID: 27565348
- Results from next generation sequencing identified minority resistance mutations in the HIV-1 integrase coding region. PMID: 26587787
- NMR characterization of HIV-1 reverse transcriptase binding to various non-nucleoside reverse transcriptase inhibitors with different activities. PMID: 26510386
- Analysis of the zidovudine resistance mutations T215Y, M41L, and L210W in HIV-1 reverse transcriptase has been presented. PMID: 26324274
- The protease-reverse transcriptase (Pro-RT) region was sequenced and analyzed for the identification of antiretroviral resistance-associated mutations (RAMs)in 40 samples. 39 samples possessed multiple RAMs in the reverse transcriptase genes. PMID: 26122980
- These results suggest that the Gag-Pol assembly and budding defects are largely due to a lack of p6gag, but also in part due to size limitation. PMID: 26489905
- RIPK1 and RIPK2 are targets of HIV-1 Protease activity during infection, and their inactivation may contribute to modulation of cell death and host defense pathways by HIV-1 PMID: 26297639
- Role of HIV-1 matrix protein p17 variants in lymphoma pathogenesis. PMID: 26578780
- these results show that the addition of R263K to the T66I substitution diminishes viral replicative capacity and strand transfer activity while not compromising susceptibility to dolutegravir. This supports the use of dolutegravir in second-line therapy for patients failing elvitegravir therapy who harbor the T66I resistance substitution. PMID: 26311878
- Together, these results provide evidence for an Integrase/Pol mediated uptake of LEDGF/p75 in HIV-1 viral particles and a specific cleavage by HIV protease. PMID: 25809198
- Study emphasizes the importance of the hydrophobic nature and SH3 fold of C-terminal domain in proper functioning of HIV integrase PMID: 25586721
- Report interaction between HIV-1 reverse transcriptase amino acid, Lys101, and a non nucleoside RT inhibitor, GW420867X. PMID: 24965933
- Design new inhibitors which could be effectively bypass the drug resistance of HIV-1 RT mutants. PMID: 25234608
- Report series of calix[4]arene-based beta-diketo derivatives that may circumvent resistance to clinical HIV-1 IN inhibitors. PMID: 25682937
- Novel 2H-pyran-2-one derivatives did not function as HIV-1 reverse transcriptase inhibitors. PMID: 25523431
- Crystal structure of MDR769 I10V HIV-1 protease co-crystallized with TLF-PafF. PMID: 25108107
- Dolutegravir resistance mutation R263K cannot coexist in combination with many classical integrase inhibitor resistance substitutions. PMID: 25653436
- Overall, this study demonstrates the novel interaction between HIV-1 integrase and cellular DYNLL1 proteins and suggests the requirement of this virus-cell interaction for proper uncoating and efficient reverse transcription of HIV-1. PMID: 25568209
- These results also indicate the importance of the RNase H N-terminal residue in the dimerization of reverse transcriptase subunits. PMID: 25392207
- REVIEW: published computer-aided structural predictions of HIV-1 integrase in complex with its interactors PMID: 24001231
- Data show that the same site on integrase-binding domain (IBD) of lens epithelium-derived growth factor (LEDGF) is involved in binding to MLL protein and HIV-IN (integrase), indicating an approach to target LEDGF in both leukemia and HIV infection. PMID: 25305204
- Aggregation assays with truncated IN variants revealed that a construct with catalytic and C-terminal domains of IN only formed an open polymer associated with efficient drug-induced aggregation. PMID: 24904063
- C-terminal residues Lys-264 and Lys-266 play an important role in the inhibitor induced aberrant multimerization of HIV-1 integrase. PMID: 25118283
- Data suggest that the HIV-1 integrase (IN)/transportin-SR2 (TRN-SR2) interaction interface is a potential target for antiviral therapy. PMID: 25063804
- The co-evolution of capsid protein and integrase sequences is related to steps of nuclear viral entry. PMID: 24474329
- This study brings the cellular and test tube results in closer agreement by showing that HIV-1 reverse transcriptase is not more error prone than other reverse transcriptases and, when assayed under physiological magnesium conditions. PMID: 24850729
- In a double drug-resistant G140S/Q148H HIV-integrase mutant, anti-HIV raltegravir spontaneously dissociates from the active site of the protein. PMID: 24205814
- The Gag-Pol ribosomal frameshift signal plays an important role in HIV-1 RNA packaging. PMID: 24453371
- Single-nucleotide polymorphisms in the 3' region of the pol gene modulate viral replication ability. PMID: 24478432
- This study supports the relevance of a rare mutation at reverse transcriptase Lys65 (K65E) in nucleos(t)ide reverse transcriptase inhibitors resistance, particularly to tenofovir. PMID: 23749955
- The presence of the N348I or M184V/N348I mutation in reverse transcriptase decreased the replication capacity of E138K virus. PMID: 24227862
- Multiple genetic pathways involving tyrosine 143 of HIV-1 integrase are preferentially associated with specific secondary amino acid substitutions and confer resistance to raltegravir and cross-resistance to elvitegravir. PMID: 23733474
- Increased risk of Q151M and K65R mutations in patients failing stavudine-containing first-line antiretroviral therapy in Cambodia. PMID: 24015311
- Data indicate that thienopyrimidinones inhibite both wild type HIV-1 reverse transcriptase (HIV RT) and drug-resistant variants, and destabilize RNase H, in some instances reducing the Tm by 5 degrees C. PMID: 23631411
- Data indicate that the stabilized peptides inhibit the interaction of HIV-1 integrase (IN) with the cellular cofactor LEDGF/p75. PMID: 23758584
- Data indicate theat the structure provides direct determination of hydrogen atom positions in the HIV-1 protease active site. PMID: 23772563
- Data suggest that the triad of Arg358, Gly359, and Ala360 in the major groove DNA/RNA-binding track of HIV-1 reverse transcriptase has major impacts on binding affinity, enzyme stability, and catalytic efficiency at high temperature. PMID: 24303887
收起更多
-
亚细胞定位:[Gag-Pol polyprotein]: Host cell membrane; Lipid-anchor. Host endosome, host multivesicular body.; [Matrix protein p17]: Virion membrane; Lipid-anchor. Host nucleus. Host cytoplasm.; [Capsid protein p24]: Virion.; [Nucleocapsid protein p7]: Virion.; [Reverse transcriptase/ribonuclease H]: Virion.; [Integrase]: Virion. Host nucleus. Host cytoplasm.
-
数据库链接:
KEGG: vg:155348