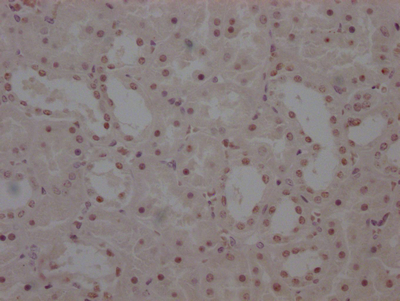
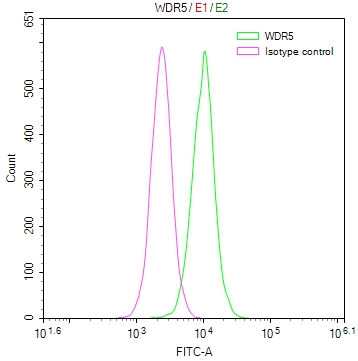
UGDH Antibody
-
货号:CSB-PA060041
-
规格:¥880
-
其他:
产品详情
-
Uniprot No.:O60701
-
基因名:UGDH
-
别名:GDH antibody; UDP Glc dehydrogenase antibody; UDP GlcDH antibody; UDP glucose 6 dehydrogenase antibody; UDP glucose dehydrogenase antibody; UDP-Glc dehydrogenase antibody; UDP-GlcDH antibody; UDP-glucose 6-dehydrogenase antibody; UDP-glucose dehydrogenase antibody; UDPGDH antibody; UGD antibody; Ugdh antibody; UGDH_HUMAN antibody; Uridine diphospho glucose dehydrogenase antibody
-
宿主:Rabbit
-
反应种属:Human,Mouse,Rat
-
免疫原:Synthesized peptide derived from the C-terminal region of Human UDP-GlcDH.
-
免疫原种属:Homo sapiens (Human)
-
标记方式:Non-conjugated
-
抗体亚型:IgG
-
纯化方式:The antibody was affinity-purified from rabbit antiserum by affinity-chromatography using epitope-specific immunogen.
-
浓度:It differs from different batches. Please contact us to confirm it.
-
保存缓冲液:Liquid in PBS containing 50% glycerol, 0.5% BSA and 0.02% sodium azide.
-
产品提供形式:Liquid
-
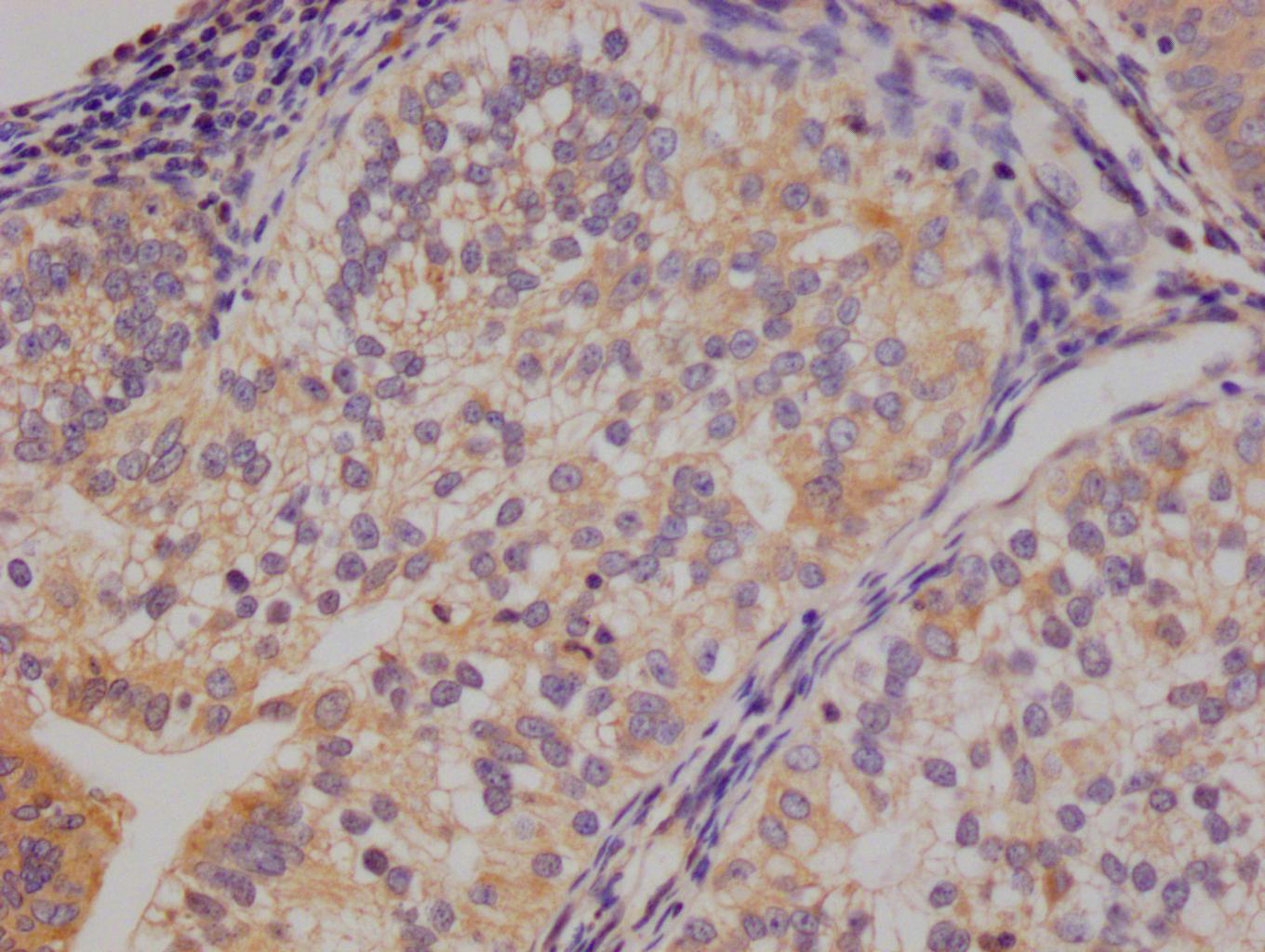
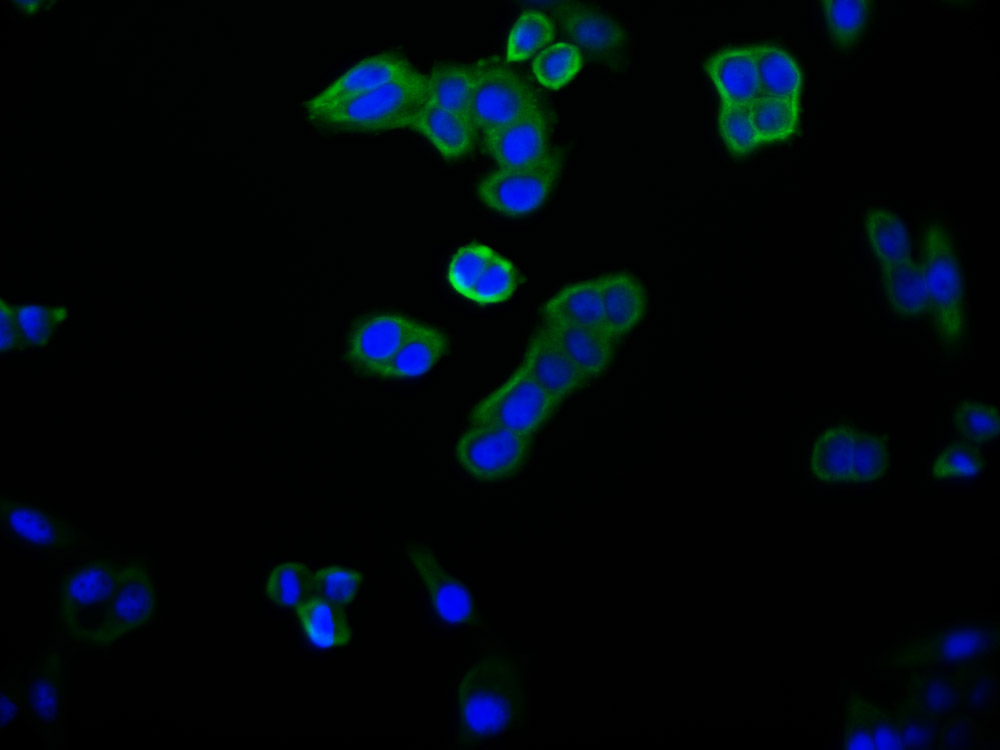
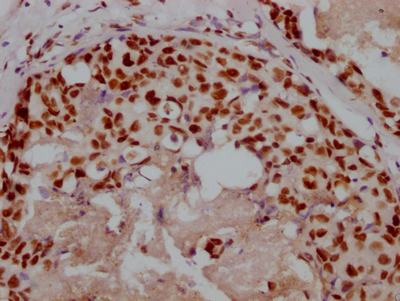
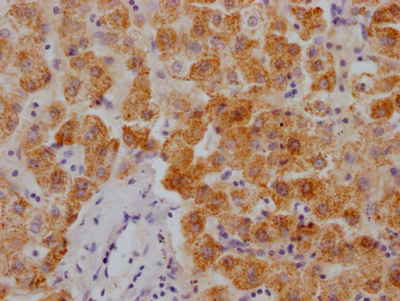
应用范围:WB, IHC, ELISA
-
推荐稀释比:
Application Recommended Dilution WB 1:500-1:2000 IHC 1:100-1:300 ELISA 1:10000 -
Protocols:
-
储存条件:Upon receipt, store at -20°C or -80°C. Avoid repeated freeze.
-
货期:Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time maybe differs from different purchasing way or location, please kindly consult your local distributors for specific delivery time.
相关产品
靶点详情
-
功能:Catalyzes the formation of UDP-alpha-D-glucuronate, a constituent of complex glycosaminoglycans. Required for the biosynthesis of chondroitin sulfate and heparan sulfate. Required for embryonic development via its role in the biosynthesis of glycosaminoglycans. Required for proper brain and neuronal development.
-
基因功能参考文献:
- Data indicate that the A136M substitution in UDP-glucose dehydrogenase (hUGDH) stabilizes the hexamer. PMID: 27966912
- study has identified several new proteins like RHOC, DLG5, UGDH, TMOD3 in addition to known chemoresistance associated proteins in non-small cell lung carcinoma. PMID: 26898345
- UGDH protein level in osteoarthritis cartilage was much lower than in control cartilage. PMID: 25465897
- UGDH displays hysteresis because of a slow isomerization from an inactive state (E*) to an active state (E). We show that the structure of E* constrains UGDH in a conformation that favors feedback inhibition at physiological pH. PMID: 25478983
- Kinetic analysis of wild-type UGDH and hexamer T325A showed that upon increasing enzyme concentration, which favors the hexameric species, activity was decreased and exhibited cooperativity. Cooperative kinetics was not observed in obligate dimer T325D. PMID: 24145036
- Mammalian UGDH displays hysteresis (observed as a lag in progress curves), indicating that the enzyme undergoes a slow transition from an inactive to an active state. Human UGDH is sensitive to product inhibition during the lag. PMID: 23363239
- both missense mutations significantly reducing the ability of UGDH to provide precursors for cardiac cushion formation, which is essential to subsequent valve formation. PMID: 22815472
- Structural and kinetic evidence that catalytic reaction of human UDP-glucose 6-dehydrogenase involves covalent thiohemiacetal and thioester enzyme intermediates. PMID: 22123821
- An alternate crystal structure of human UGDH (hUGDH) in complex with UDP-glucose at 2.8 A resolution, is reported. PMID: 21984906
- A structurally detailed model of UDP-alpha-D-glucose 6-dehydrogenase (UGDH) demonstrates hinge-bending motion that represents allosteric feedback inhibition and substrate-product exchange during the catalytic cycle. PMID: 21961565
- high UGDH levels may underlie susceptibility of the orbit to localized overproduction of hyaluronan in Graves disease. PMID: 21576248
- Structure and mechanism of human UDP-glucose 6-dehydrogenase. PMID: 21502315
- An atypical allosteric mechanism in human UDP-alpha-D-glucose 6-dehydrogenase (UGDH) based on an easily acquired and identifiable structural attribute: packing defects in the protein core. PMID: 21595445
- UGDH can regulate cell motility through the production of glycosaminoglycans PMID: 20691680
- Results support the UGDH content in prostatic acini as a novel candidate biomarker that may complement the development of a multi-biomarker panel for detecting PC within the tumor adjacent field on a histologically normal biopsy specimen. PMID: 19676054
- UGDH core promoter has a role in up- and down-regulation of UGDH after TGF-beta stimulation and in hypoxic conditions PMID: 12682078
- role of Cys-276 as a catalytic residue; Lys-279 is likely to have a role in positioning active site residues and in maintaining the hexameric quaternary structure PMID: 15044486
- Gly-13 plays an important role for efficient binding of NAD(+) to human UDP-glucose dehydrogenase PMID: 15247292
- C276 plays an important role for efficient binding of UDP-glucose to hUGDH PMID: 15898741
- Our results indicate that the region from nucleotide position -486 to -632 relative to the start of the small transcript contains positive regulatory elements that contribute to gene expression. PMID: 16002992
- report the presence of an inhibitory cis-element in the distal region of the UGDH promoter that interacts with putative transcriptional repressors for the negative regulation of the UGDH gene PMID: 16495656
- UGDH was purified and crystallized, and diffraction data proposes that the biological unit of UGDH is a tetramer. PMID: 17073734
- Alteration of lysine 220 to alanine, histidine, or arginine significantly impaired enzyme function. PMID: 17209547
- Results suggest that UGDH Ala222 and Ser233 play an important role in maintaining the hexameric structure and the reduced binding affinities for substrates are attributable to its altered subunit communication. PMID: 17927902
- This demonstrates that the UGDH transcript and protein quantities, the enzyme activity, and glycosaminoglycan contents increase in LMP2A overexpressed human embryonic kidney 293 (HEK293) cells. PMID: 18717819
- perturbation caused by the mutation of a residue at a considerably distant location from the oligomeric interfaces is preferentially distributed throughout specific sites, especially the large flexible regions in the UGDH structure PMID: 19358821
显示更多
收起更多
-
蛋白家族:UDP-glucose/GDP-mannose dehydrogenase family
-
组织特异性:Detected in heart, placenta, liver, pancreas, spleen, thymus, prostate, ovary, small intestine and colon. Widely expressed.
-
数据库链接:
HGNC: 12525
OMIM: 603370
KEGG: hsa:7358
STRING: 9606.ENSP00000319501
UniGene: Hs.572518
Most popular with customers
-
-
YWHAB Recombinant Monoclonal Antibody
Applications: ELISA, WB, IF, FC
Species Reactivity: Human, Mouse, Rat
-
Phospho-YAP1 (S127) Recombinant Monoclonal Antibody
Applications: ELISA, WB, IHC
Species Reactivity: Human
-
-
-
-
-