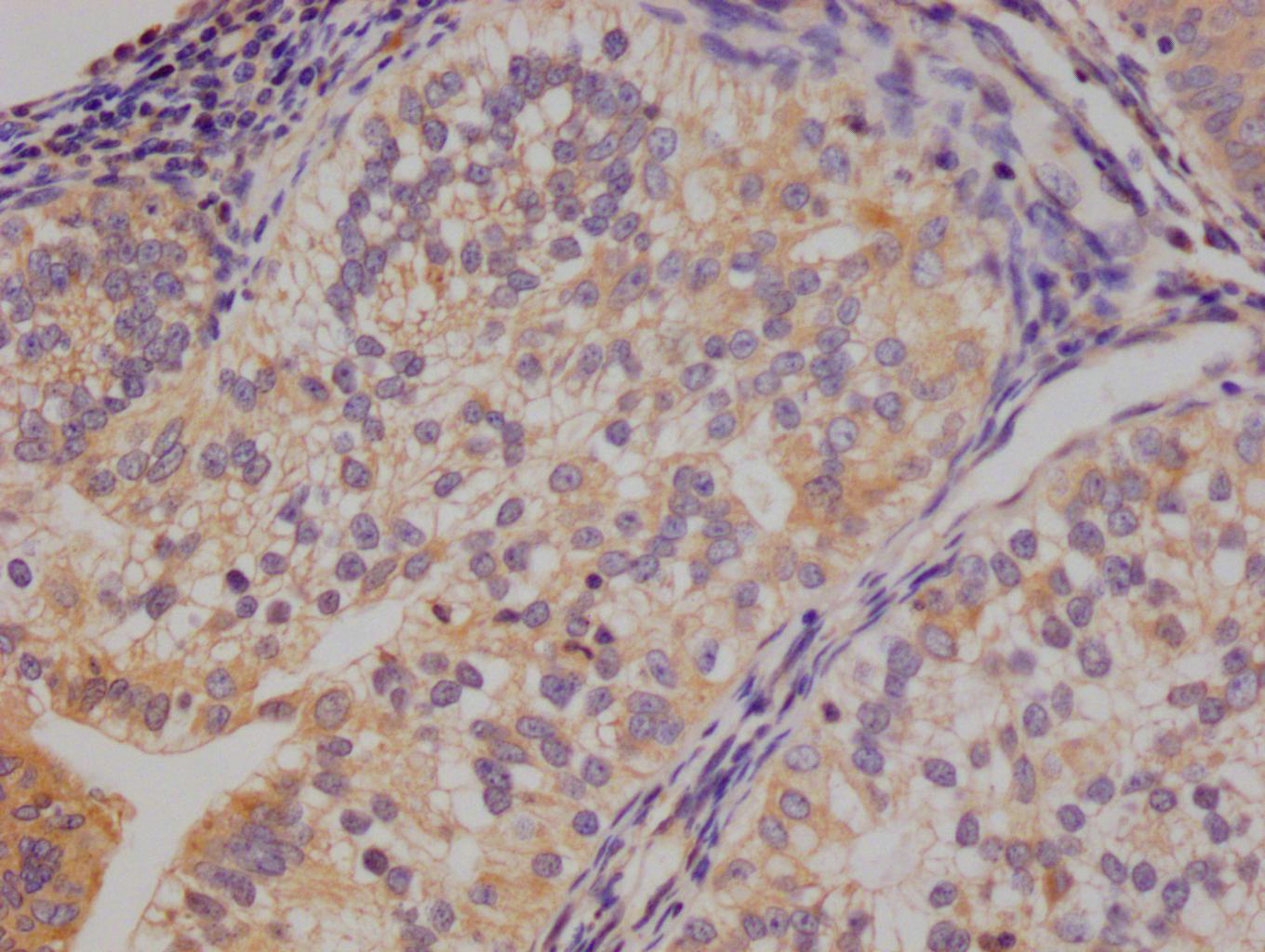
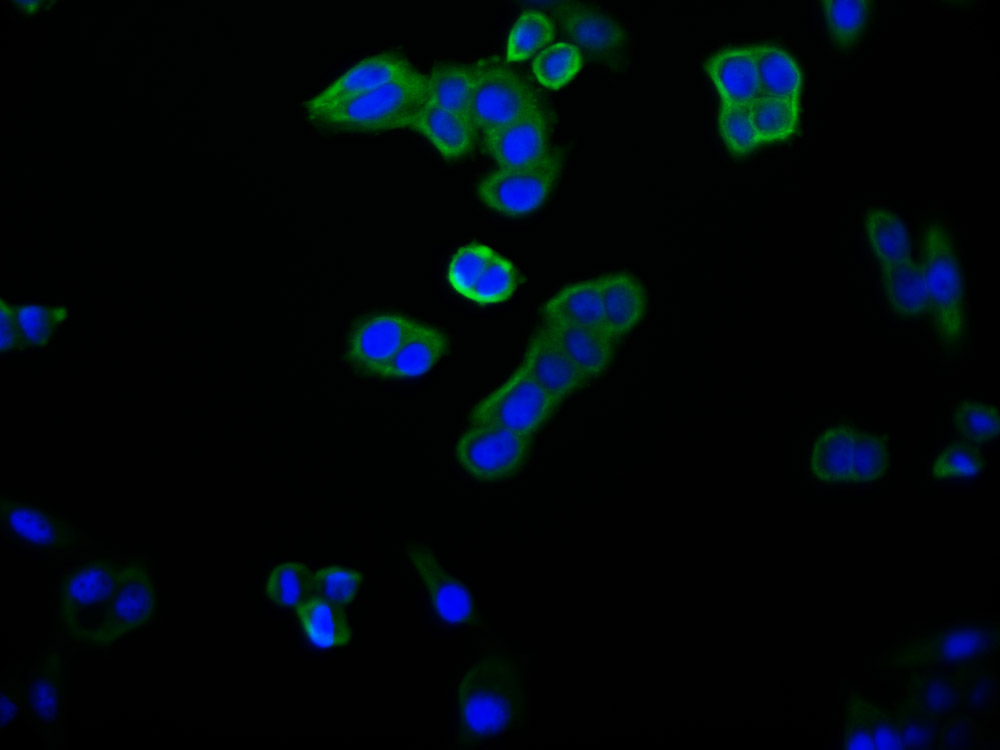
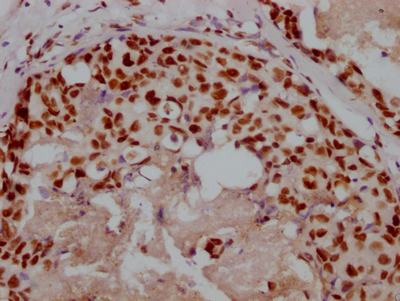
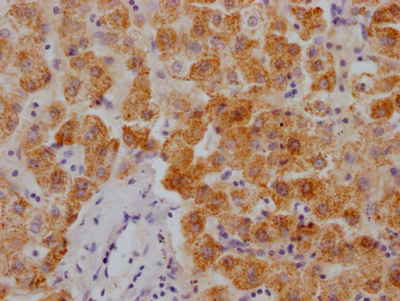
SPTA1 Antibody
-
货号:CSB-PA966552
-
规格:¥880
-
其他:
产品详情
-
产品名称:Rabbit anti-Homo sapiens (Human) SPTA1 Polyclonal antibody
-
Uniprot No.:P02549
-
基因名:
-
别名:Alpha I spectrin antibody; EL 2 antibody; EL2 antibody; Elliptocytosis 2 antibody; Elliptocytosis2 antibody; Erythrocyte alpha spectrin antibody; erythrocyte antibody; Erythroid alpha spectrin antibody; Erythroid alpha-spectrin antibody; Erythroid spectrin alpha antibody; HPP antibody; HS3 antibody; Spectrin alpha chain antibody; Spectrin alpha chain erythrocyte antibody; Spectrin alpha erythrocytic 1 antibody; SPH3 antibody; SPTA 1 antibody; SPTA antibody; SPTA1 antibody; SPTA1_HUMAN antibody
-
宿主:Rabbit
-
反应种属:Human, Mouse
-
免疫原:Synthetic peptide of Human SPTA1
-
免疫原种属:Homo sapiens (Human)
-
标记方式:Non-conjugated
-
克隆类型:Polyclonal
-
抗体亚型:IgG
-
纯化方式:Antigen Affinity Purified
-
浓度:It differs from different batches. Please contact us to confirm it.
-
保存缓冲液:PBS with 0.05% sodium azide, 40% glycerol, pH7.4
-
产品提供形式:Liquid
-
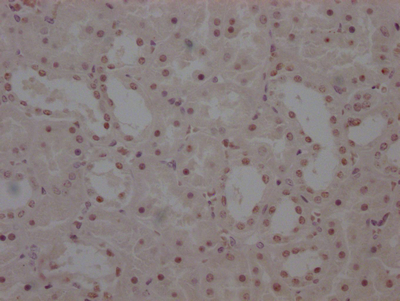
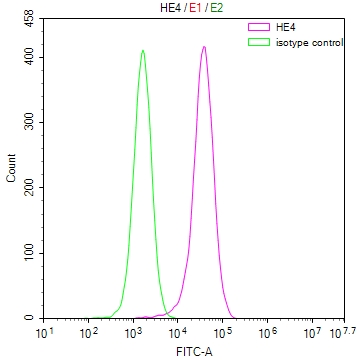
应用范围:ELISA, WB, IHC
-
推荐稀释比:
Application Recommended Dilution WB 1:200-1:1000 IHC 1:25-1:100 -
Protocols:
-
储存条件:Upon receipt, store at -20°C or -80°C. Avoid repeated freeze.
-
货期:Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time maybe differs from different purchasing way or location, please kindly consult your local distributors for specific delivery time.
相关产品
靶点详情
-
功能:Spectrin is the major constituent of the cytoskeletal network underlying the erythrocyte plasma membrane. It associates with band 4.1 and actin to form the cytoskeletal superstructure of the erythrocyte plasma membrane.
-
基因功能参考文献:
- a novel HE case with a His54Pro mutation in the SPTA1 gene was reported. The results suggested that the His54Pro mutation influenced the role of erythrocyte membrane proteins without reducing its level of expression. PMID: 29484404
- A novel SPTA1 mutation (H54P) was identified in a case of hereditary elliptocytosis. PMID: 28694211
- The s show that SUB1-mediated processing of MSP1 is important for parasite viability, the processing modifies the secondary structure of MSP1 and activates its capacity to bind spectrin. PMID: 26468747
- The s demonstrate that the initial vacuolar membrane around internalized Babesia divergens is formed from protein and lipid components of the red blood cells plasma membrane, including band 3, glycophorin A and spectrin. PMID: 25628009
- a new function for spectrins in the stability of invadosomes and the coupling between actin regulation and ECM degradation PMID: 25830635
- Case Report: severe hemolytic jaundice and a phenotype of hereditary spherocytosis due alpha-spectrin mutations. PMID: 25277063
- In this review, we summarize the state of knowledge about interactions between spectrin and membrane lipids PMID: 24569979
- A novel exon 2 alpha spectrin mutation is identified in two families of European ancestry with hereditary pyropoikilocytosis. PMID: 24077844
- The heterozygous c.121C>T mutation of SPTA1 gene induces an amino acid change p.Arg41Trp in the alpha1 domain of the alpha-spectrin protein. PMID: 24003435
- Data show that transcription cofactor TAF3 is required for transcription of the alpha spectrin SPTA1 gene. PMID: 23935956
- The common hereditary elliptocytosis-associated alpha-spectrin leucine260proline mutation perturbs erythrocyte membranes by stabilizing spectrin in the closed dimer conformation. PMID: 23974198
- The unusually slow, two-state kinetics of spectrin assembly in solution, was investigated. PMID: 23200054
- In this review, we summarize recent findings concerning structure and function of spectrin together with its possible role in pathology. PMID: 23373410
- These data further suggest that residues 44 and 53, which are key players in the nucleation-condensation mechanism of folding, are also important triggers of the aggregation process. PMID: 22727745
- analysis of glycosylation of erythrocyte spectrin and its modification in visceral leishmaniasis PMID: 22164239
- Further studies involving siRNA-mediated knockdowns of spectrin, adducin, or p4.1 revealed that those proteins are needed for efficient docking of enterohaemorrhagic Escherichia coli to host cells. PMID: 22197999
- Results suggest that it is possible for cellular proteins to differentially associate with the C-termini of different beta-spectrin isoforms to regulate alpha- and beta-spectrin association to form functional spectrin tetramers. PMID: 21412925
- lipid rafts are associated with the spectrin skeleton in human erythrocytes PMID: 20807499
- The data show that the alpha-spectrin EF domain greatly amplifies the function of the beta-spectrin actin-binding domain in forming the spectrin-actin-4.1R complex. PMID: 20585040
- The functional roles of residues 21-43 and 55-59 in the alpha-spectrin N-terminal region in forming tetramers were determined;mutations may also introduce abnormalities to erythrocytes. PMID: 19747366
- identification and characterization of the gene promoter; requires GATA-1- and NF-E2-binding proteins to direct high level expression in erythroid cells in vitro. PMID: 12196550
- Quantitative analysis of erythrocyte membrane proteins revealed increase in alpha-spectrin from patients with homozygous and heterozygous forms of beta-thalassemia. PMID: 15310273
- a region 3' of the alpha-spectrin core promoter contains a GATA-1-dependent positive regulatory element that is required in its proper genomic orientation PMID: 15456760
- analysis of erythroid alpha and beta spectrin chaperone activity and prodan binding PMID: 15492010
- Ubiquitination of alpha-spectrin does not regulate heterodimer formation. PMID: 15795915
- splicing mutation in hereditary pyropoikilocytosis kindred PMID: 16150946
- We found that cysteine 2071 & cysteine 2100 are critical for alpha-spectrin (2005-2415) E2/E3 activity; also demonstrated that both Cys2071 & Cys2100 are capable of transferring ubiquitin from an E1 enzyme to target sites within alpha-spectrin (2005-2415) PMID: 16171554
- the interaction of the alphaII-spectrin SH3 domain with EVL PMID: 16336193
- These results suggest a role for spectrin in providing a dynamic and reversible signaling platform to the specific domains of the plasma membrane in response to stimulation of GPCR. PMID: 16551696
- Results provide evidence that protein degradation of alphaII-spectrin is a reliable marker of severe traumatic brain injury (TBI) in humans and that both necrotic and apoptotic cell death mechanisms are activated in humans following a severe TBI. PMID: 16841024
- REVIEW: Culture studies of Plasmodium falciparum in elliptocytes bearing such elliptocytogenic alleles of spectrin showed that these alleles are supplementary genetic factors of malaria resistance PMID: 17414207
- The absence of particular spectrin isoforms may correlate with transformation or aggressive biologic behavior for some lymphoma subtypes. PMID: 17885671
- analysis of the conformational change of erythroid alpha-spectrin at the tetramerization site upon binding beta-spectrin PMID: 17905835
- This model supports the hypothesis that initial docking of the correct alpha and beta repeats from among many very similar repeats in both subunits is driven primarily by long range electrostatic interactions. PMID: 17977835
- All alpha0 HE/HPP mutations studied here appear to exert their destabilizing effects through molecular recognition rather than structural mechanisms. PMID: 18218854
- Erythrocytes from most jereditary pyropoikilocytosis (inherited hemolytic anemia) exhibit abnormalities in the alpha-spectrin gene. PMID: 18815189
- exon 1' and intron 1' are excellent candidate regions for mutations in patients with spectrin-linked hemolytic anemia PMID: 19008453
- The L49F mutation in alpha erythroid spectrin leads to an unstable triple helical bundle of alpha beta-spectrin partial domains, and thus unstable tetramers. PMID: 19593814
显示更多
收起更多
-
相关疾病:Elliptocytosis 2 (EL2); Hereditary pyropoikilocytosis (HPP); Spherocytosis 3 (SPH3)
-
亚细胞定位:Cytoplasm, cytoskeleton. Cytoplasm, cell cortex.
-
蛋白家族:Spectrin family
-
数据库链接:
HGNC: 11272
OMIM: 130600
KEGG: hsa:6708
STRING: 9606.ENSP00000357130
UniGene: Hs.119825
Most popular with customers
-
-
YWHAB Recombinant Monoclonal Antibody
Applications: ELISA, WB, IF, FC
Species Reactivity: Human, Mouse, Rat
-
Phospho-YAP1 (S127) Recombinant Monoclonal Antibody
Applications: ELISA, WB, IHC
Species Reactivity: Human
-
-
-
-
-