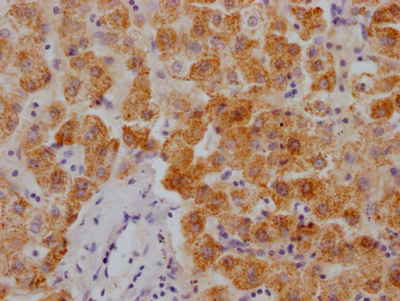
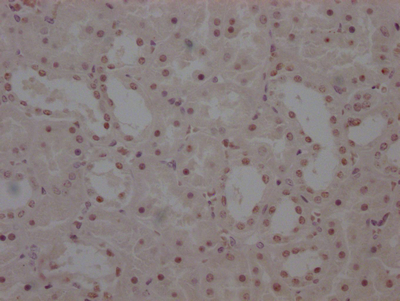
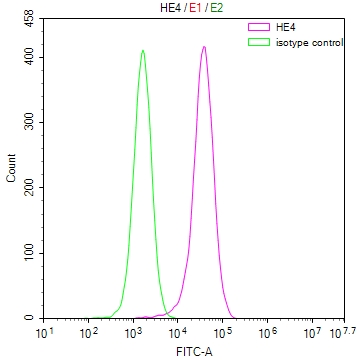
SERPINI1 Antibody
-
货号:CSB-PA869434
-
规格:¥2024
-
图片:
-
其他:
产品详情
-
产品名称:Rabbit anti-Homo sapiens (Human) SERPINI1 Polyclonal antibody
-
Uniprot No.:Q99574
-
基因名:SERPINI1
-
宿主:Rabbit
-
反应种属:Human,Mouse,Rat
-
免疫原:Peptide sequence around aa.402~406(T-S-G-H-D) derived from Human Neuroserpin.
-
免疫原种属:Homo sapiens (Human)
-
克隆类型:Polyclonal
-
纯化方式:Antibodies were produced by immunizing rabbits with synthetic peptide and KLH conjugates. Antibodies were purified by affinity-chromatography using epitope-specific peptide.
-
浓度:It differs from different batches. Please contact us to confirm it.
-
产品提供形式:Liquid
-
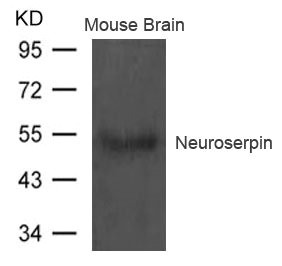
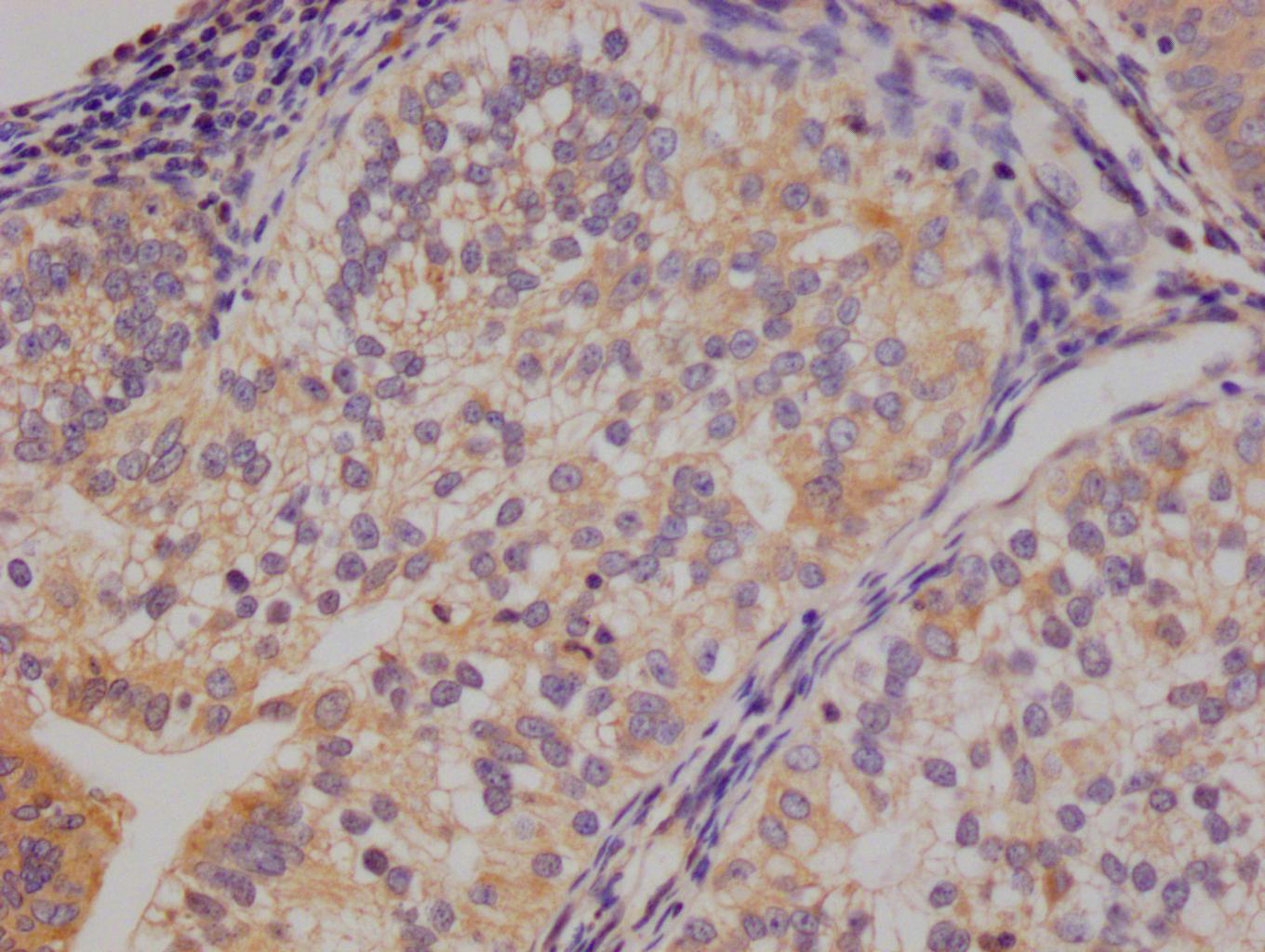
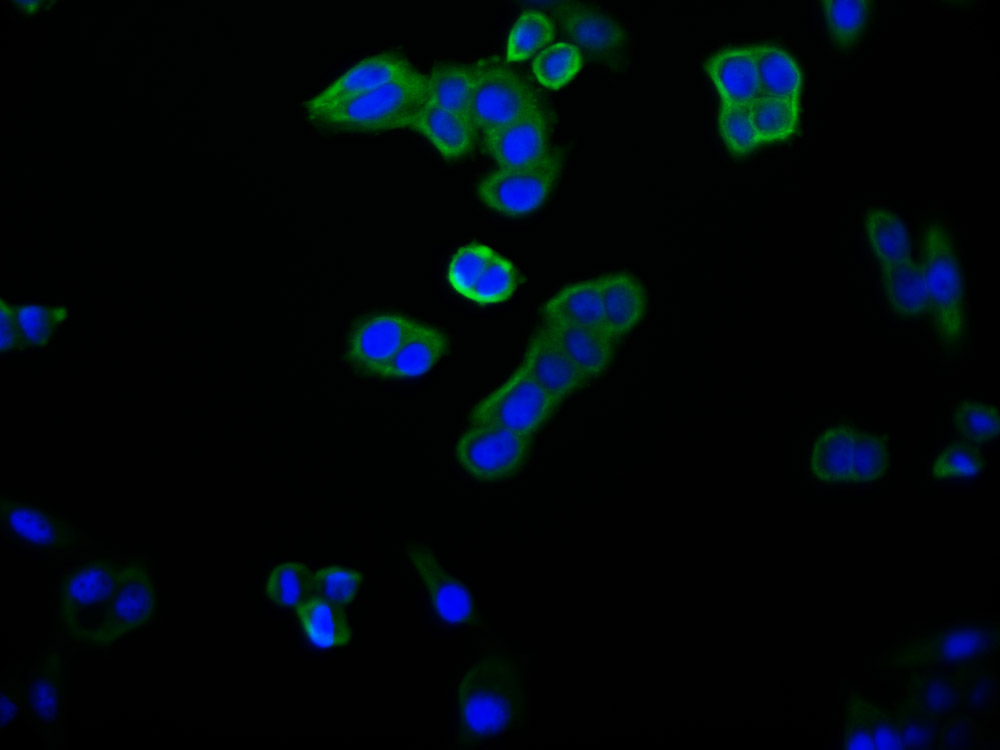
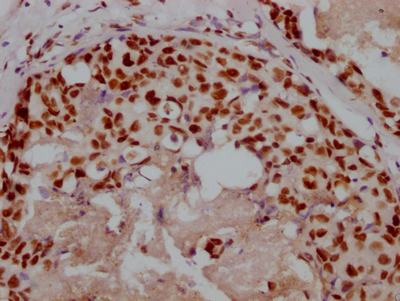
应用范围:ELISA,WB
-
推荐稀释比:
Application Recommended Dilution WB 1:500-1:1000 -
Protocols:
-
储存条件:Upon receipt, store at -20°C or -80°C. Avoid repeated freeze.
-
货期:Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time maybe differs from different purchasing way or location, please kindly consult your local distributors for specific delivery time.
相关产品
靶点详情
-
功能:Serine protease inhibitor that inhibits plasminogen activators and plasmin but not thrombin. May be involved in the formation or reorganization of synaptic connections as well as for synaptic plasticity in the adult nervous system. May protect neurons from cell damage by tissue-type plasminogen activator (Probable).
-
基因功能参考文献:
- We present two pediatric cases of progressive myoclonic epilepsy with SERPINI1 pathogenic variants that lead to a severe presentation. PMID: 28631894
- Data indicated that rs9853967 and rs11714980 polymorphisms in CCM3 and SERPINI1respectively could be associated with a protective role in cerebral cavernous malformations disease. PMID: 27737651
- SERPINI1 is an important regulator of epithelial-mesenchymal transition in an orthotopic implantation model of colorectal cancer PMID: 26892864
- The thermal and chemical stability along with the polymerisation propensity of both Wild Type and Glu289Ala NS were characterized. PMID: 26329378
- This C-terminal lability is not required for neuroserpin polymerisation in the endoplasmic reticulum, but the additional glycan facilitates degradation of the mutant protein during proteasomal impairment. PMID: 26367528
- the protective effect of neuroserpin maybe independent from its canonical interaction with a tissue-type plasminogen activator PMID: 26176694
- Neuroserpin is expressed in naive effector memory and central memory CD4 and CD8 T cell subsets, and monocytes, B cells, and NK cells. T-cell activation caused its translocation to the immunologic synapse, secretion, and delayed downregulation. PMID: 25670787
- Molecular Dynamics simulations suggest that Neuroserpin conformational stability and flexibility arise from a spatial distribution of intramolecular salt-bridges and hydrogen bonds. PMID: 25450507
- Alzheimer's disease brain tissues with elevated neuroserpin protein also showed increased expression of THRbeta1 and HuD PMID: 24036060
- our study did not provide any evidence for an association between genetic variation at the SERPINI1 locus and ischemic stroke PMID: 21487809
- the origins of conformational lability PMID: 21961602
- Neuroprotective properties of neuroserpin may be related to the inhibition of excitotoxicity, inflammation, as well as blood brain barrier disruption that occur after acute ischemic stroke. PMID: 21569344
- Hrd1 and gp78 mediate mutant neuroserpin turnover through the ERAD pathway. PMID: 21507957
- high serum neuroserpin levels before intravenous tPA and neuroserpin levels decrease at 24 h after ischaemic stroke, independently of tPA treatment, may have a role in good functional outcome PMID: 21174006
- The latent and polymer hNS forms obtained at 45 degrees C and 85 degrees C differ in their chemical and thermal stabilities; furthermore, the human neuroserpin polymers also differ in size and morphology PMID: 21081089
- investigated the refolding and polymerization pathways of wild-type neuroserpin and of the pathogenic mutants S49P and H338R PMID: 20691191
- Mutant Neuroserpin (S49P) that causes familial encephalopathy with neuroserpin inclusion bodies is a poor proteinase inhibitor and readily forms polymers in vitro PMID: 11880376
- The interactions between NSP and t-PA were distinct from those between plasmin and NSP, suggesting that the physiologic effect of t-PA-NSP interactions may be more complex than previously thought. PMID: 12228252
- Neuroserpin has a role as a selective inhibitor of tissue-type plasminogen activator in the central nervous system [review] PMID: 14983220
- neuroserpin mutants that cause dementia accumulate as polymers within the endoplasmic reticulum PMID: 15090543
- tissue plasminogen activator and neuroserpin are widely expressed in the human central nervous system PMID: 15269833
- reactive centre loop of neuroserpin Portland being partially inserted into beta-sheet A to adopt a conformation similar to an intermediate on the polymerization pathway PMID: 15291813
- Data show that the S49P mutant of neuroserpin that causes the dementia familial encephalopathy with neuroserpin inclusion bodies (FENIB) forms a latent species in vitro and in vivo in addition to the formation of polymers. PMID: 15664988
- neuroserpin interacts with Abeta(1-42) to form off-pathway non-toxic oligomers and so protects neurons in Alzheimer disease PMID: 16849336
- intergenic region of the head-to-head PDCD10-SERPINI1 gene pair provides an interesting and informative example of a complex regulatory system PMID: 17212813
- in a French family with the S52R mutation of the neuroserpin gene, progressive myoclonic epilepsy was associated with a frontal syndrome PMID: 17606885
- This study provides the first evidence that neuroserpin is associated with early-onset ischemic stroke among Caucasian women. PMID: 17961231
- conformational modification in the protein under oxidative stress PMID: 18051703
- We report a neuroserpin mutation that causes electrical status epilepticus of slow-wave sleep. PMID: 18591508
- Neuroserpin and tissue plasminogen activator are associated with amyloid-beta plaques in Alzheimer brain tissue. PMID: 19222708
- Human neuroserpin: structure and time-dependent inhibition PMID: 19265707
- Analyses restricted to glioblastoma (n = 254) yielded significant associations for the SELP, DEFB126/127, SERPINI1, and LY96 genetic regions. PMID: 19423540
- intracellular neuroserpin polymers activate NF-kappaB by a pathway that is independent of the IRE1, ATF6, and PERK limbs of the canonical unfolded protein response but is dependent on intracellular calcium PMID: 19423713
显示更多
收起更多
-
相关疾病:Encephalopathy, familial, with neuroserpin inclusion bodies (FENIB)
-
亚细胞定位:Secreted. Cytoplasmic vesicle, secretory vesicle lumen. Perikaryon.
-
蛋白家族:Serpin family
-
组织特异性:Detected in brain cortex and hippocampus pyramidal neurons (at protein level). Predominantly expressed in the brain.
-
数据库链接:
HGNC: 8943
OMIM: 602445
KEGG: hsa:5274
STRING: 9606.ENSP00000295777
UniGene: Hs.478153
Most popular with customers
-
-
YWHAB Recombinant Monoclonal Antibody
Applications: ELISA, WB, IF, FC
Species Reactivity: Human, Mouse, Rat
-
Phospho-YAP1 (S127) Recombinant Monoclonal Antibody
Applications: ELISA, WB, IHC
Species Reactivity: Human
-
-
-
-
-