NPC1 Antibody
-
货号:CSB-PA948316
-
规格:¥1100
-
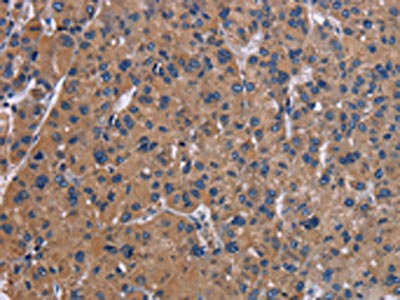
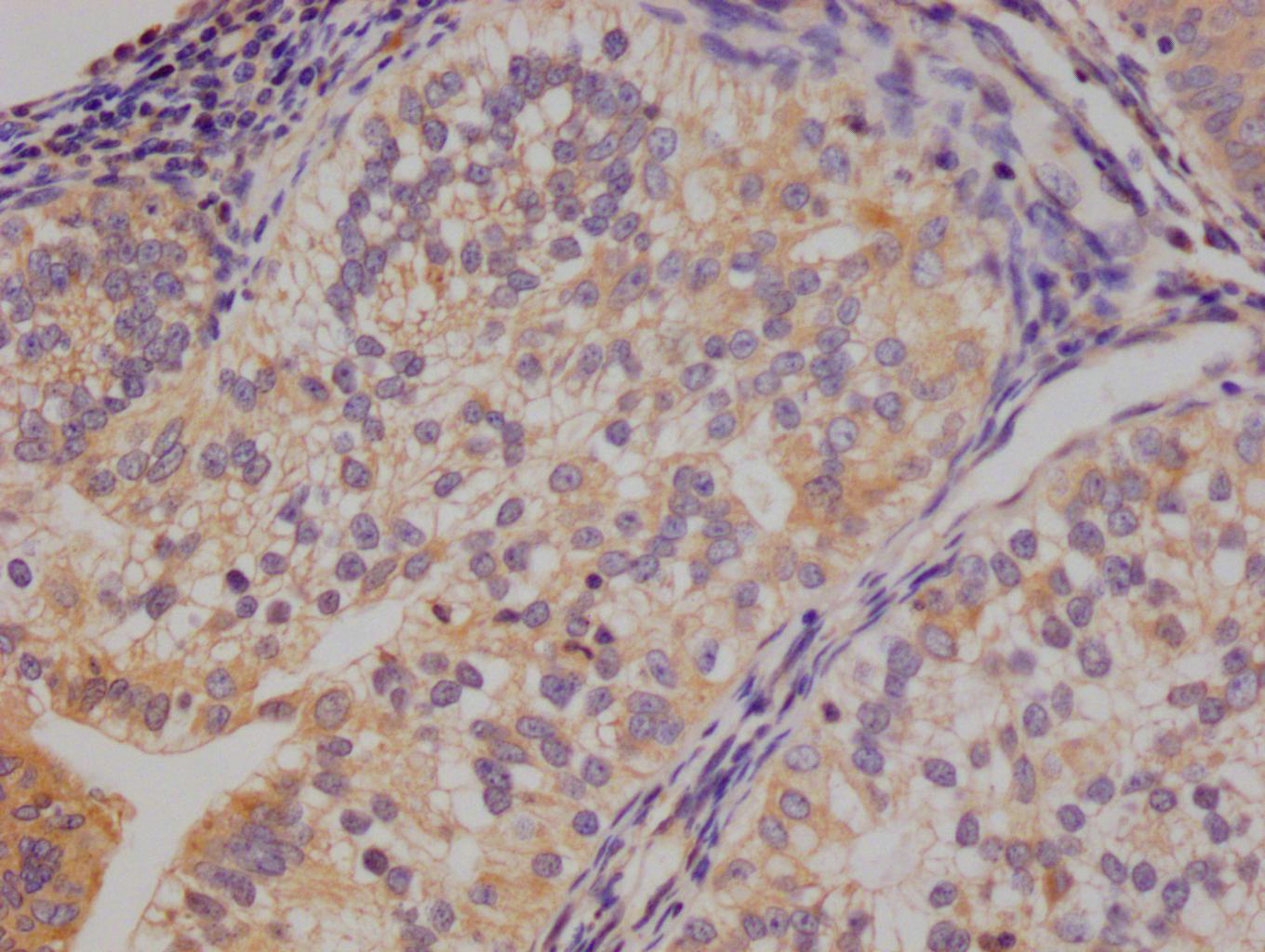
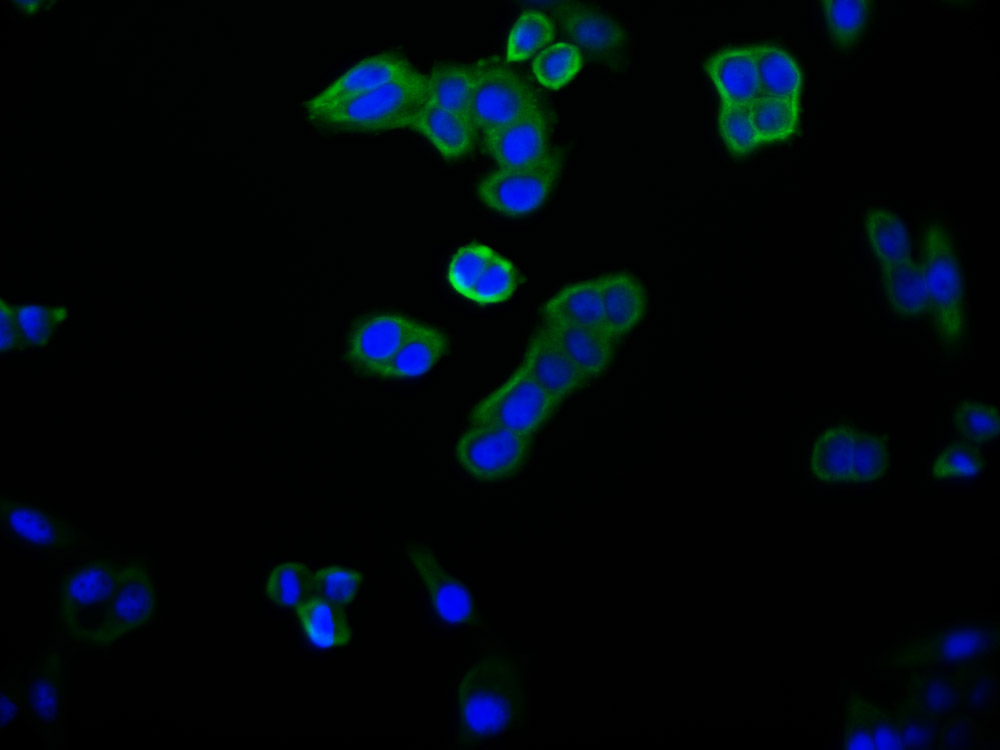
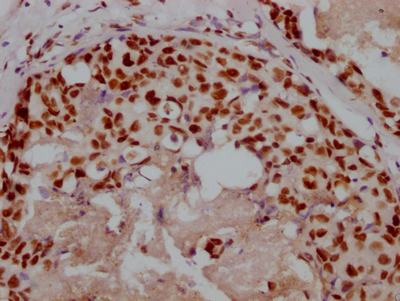
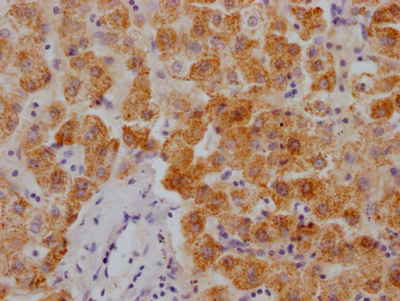
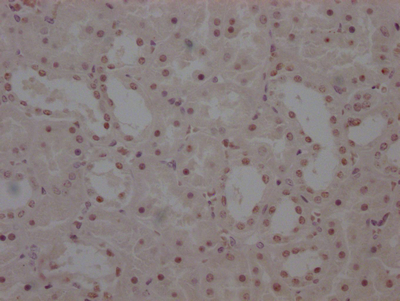
图片:
-
其他:
产品详情
-
Uniprot No.:O15118
-
基因名:
-
别名:Niemann Pick C1 protein precursor antibody; Niemann Pick disease, type C1 antibody; Niemann-Pick C1 protein antibody; NPC antibody; NPC1 antibody; NPC1_HUMAN antibody
-
宿主:Rabbit
-
反应种属:Human
-
免疫原:Synthetic peptide of Human NPC1
-
免疫原种属:Homo sapiens (Human)
-
标记方式:Non-conjugated
-
抗体亚型:IgG
-
纯化方式:Antigen affinity purification
-
浓度:It differs from different batches. Please contact us to confirm it.
-
保存缓冲液:-20°C, pH7.4 PBS, 0.05% NaN3, 40% Glycerol
-
产品提供形式:Liquid
-
应用范围:ELISA,IHC
-
推荐稀释比:
Application Recommended Dilution ELISA 1:1000-1:2000 IHC 1:25-1:100 -
Protocols:
-
储存条件:Upon receipt, store at -20°C or -80°C. Avoid repeated freeze.
-
货期:Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time maybe differs from different purchasing way or location, please kindly consult your local distributors for specific delivery time.
相关产品
靶点详情
-
功能:Intracellular cholesterol transporter which acts in concert with NPC2 and plays an important role in the egress of cholesterol from the endosomal/lysosomal compartment. Unesterified cholesterol that has been released from LDLs in the lumen of the late endosomes/lysosomes is transferred by NPC2 to the cholesterol-binding pocket in the N-terminal domain of NPC1. Cholesterol binds to NPC1 with the hydroxyl group buried in the binding pocket. Binds oxysterol with higher affinity than cholesterol. May play a role in vesicular trafficking in glia, a process that may be crucial for maintaining the structural and functional integrity of nerve terminals (Probable).; (Microbial infection) Acts as an endosomal entry receptor for ebolavirus.
-
基因功能参考文献:
- Results propose that, depending on the location of the cholesterol ligand, a dynamical interface between the NPC2 and NPC1 N-terminal domain (NTD) proteins exists. Structural features of a particular interface can lower the energy barrier and stabilize the passage of the cholesterol substrate from NPC2 to NPC1(NTD). PMID: 30181526
- These data support the hypothesis that cholesterol is transported through interactions between two or more NPC1 molecules. PMID: 30047864
- Mutation in the NPC1 gene is associated with Niemann-Pick type C. PMID: 28167839
- pronounced alterations in several proteins linked to autophagy and lysosomal catabolism reflecting vesicular transport obstruction and defective lysosomal turnover resulting from NPC1 deficiency, were observed. PMID: 28134274
- Niemann-Pick C1 (NPC1) protein structures suggest mapping of all of the disease-causing mutations for future molecular insights into the pathogenesis of Niemann-Pick type C disease (NPC) disease. PMID: 28784760
- This study demonistrated that heterozygous mutations of NPC1 genes could contribute to dementia plus, at least in a subset of patients. PMID: 27792009
- Docking of the NPC1-NPC2 complex onto the full-length NPC1 structure reveals a direct cholesterol transfer tunnel between NPC2 and N-terminal domain cholesterol binding pockets, supporting the "hydrophobic hand-off" cholesterol transfer model. PMID: 27551080
- Taken together, these studies suggest that Ebola virus requires phosphatidylinositol (3,5) bisphosphate production in cells to promote efficient delivery to NPC1. PMID: 29031163
- identification of NPC1 and/or NPC2 mutations combined with descriptions of clinical phenotype, will improve our knowledge of pathogenic mutations and our understanding of genotype-phenotype correlations. PMID: 27339554
- Here we report a crystal structure of a large fragment of human NPC1 at 3.6 A resolution, which reveals internal twofold pseudosymmetry along TM 2-13 and two structurally homologous domains that protrude 60 A into the endosomal lumen, and we propose a model for NPC1 function in cholesterol sensing and transport. PMID: 27307437
- Sequencing of genomic DNA from GM03123 Led to the identification of a mutation in NPC1 GENE, g.41940G>C (c.1947 + 5G>C; rs770321568) (Fig. 1A), with a minor allele frequency of 0.0000082 PMID: 28328115
- this case provides support for the V950M variant being sufficient for adult-onset Niemann-Pick type C disease. PMID: 27900365
- We identified major events in NPC1 evolution and revealed and compared orthologs and paralogs of the human NPC1 gene through phylogenetic and protein sequence analyses. We predicted whether an amino acid substitution affects protein function by reducing the organism's fitness. PMID: 26890452
- The mutant NPC1 did not significantly reduce cholesterol accumulation, but approximately 85% of the mutants showed reduced cholesterol accumulation when treated with vorinostat or panobinostat. PMID: 28193631
- knockdown of TMEM97 also increases levels of residual NPC1 in NPC1-mutant patient fibroblasts and reduces cholesterol storage in an NPC1-dependent manner. Our findings propose TMEM97 inhibition as a novel strategy to increase residual NPC1 levels in cells and a potential therapeutic target for Niemann-Pick type C disease (NP-C). PMID: 27378690
- The splicing mutation IVS23 + 3insT was associated in homozygocity with a severe biochemical and clinical phenotype. A possible founder effect for this mutation was demonstrated in the Greek Island, as well as a different origin for each novel mutation PMID: 28472934
- Rare loss-of-function NPC1 mutations were identified as being associated with human adiposity with a high penetrance in a Chinese population. PMID: 28130309
- Furthermore saturation and intracellular distribution of alpha-Toc seem to be strongly dependent on the availability of this vitamin as well as on the presence of the lysosomal protein NPC1 PMID: 27095633
- Two mutations were identified in the NPC1 gene, one of which was novel and its pathogenetic nature was unknown PMID: 26790753
- Our data suggest an incidence rate for NPC1 and NPC2 of 1/92,104 and 1/2,858,998, respectively. Evaluation of common NPC1 variants, however, suggests that there may be a late-onset NPC1 phenotype with a markedly higher incidence. PMID: 25764212
- Fibroblasts from Niemann-Pick type C (NPC) disease patients with low levels of NPC1 protein have high amounts of procathepsin D but reduced quantities of the mature protein, thus showing a diminished cathepsin D activity. PMID: 26507101
- Results identified six novel mutations (PKHD1: p.Thr777Met, p.Tyr2260Cys; ABCB11: p.Val1112Phe, c.611+1G > A, p.Gly628Trpfs*3 and NPC1: p.Glu391Lys) for the diagnostic of inherited infantile cholestatic disorders. PMID: 25771912
- NPC1 mutations are substantially enriched in unexplained early onset ataxia, making it high risk group for Niemann-Pick disease type C. PMID: 26338816
- these results clearly demonstrated that the over-expression of NPC1 with a defective function in an imatinib-resistant Ph+ acute lymphoblastic leukemia cell line PMID: 26818574
- Structure of glycosylated NPC1 luminal domain C reveals insights into NPC2 and Ebola virus interactions PMID: 26846330
- Study determined the crystal structure of the primed GP (GPcl) of Ebola virus bound to domain C of NPC1 (NPC1-C); NPC1-C utilizes two protruding loops to engage a hydrophobic cavity on head of GPcl. Upon enzymatic cleavage and NPC1-C binding, conformational change in the GPcl further affects the state of the internal fusion loop, triggering membrane fusion. PMID: 26771495
- Here, using live cell imaging, the s obtained evidence that in contrast to the new model, ebolavirus enters cells through endolysosomes that contain both NPC1 and TPC2. PMID: 26468524
- These experiments support a model in which NPC1 protein functions to transfer cholesterol past a lysosomal glycocalyx. PMID: 26578804
- An isobaric labeling-based quantitative analysis of proteome of NPC1(I1061T) primary fibroblasts when compared with wild-type cells identified 281 differentially expressed proteins based on stringent data analysis criteria, is reported. PMID: 25873482
- NPC1 gene sequencing revealed that he was a compound heterozygote for the p.S954L and p.N1156S mutations. PMID: 25238906
- results uncover Akt as a key regulator of NPC1 degradation and link NPC1 to cancer cell proliferation and migration. PMID: 26283546
- heterozygous mutations in the NPC1/2 gene might be a risk factor for Alzheimer's disease PMID: 25220527
- In a transgenic mouse model, human NPC1 disease was faithfully recapitulated in a NPC1 I1061T mutation knock-in model. PMID: 26019327
- This study showed that Niemann-Pick C1 (NPC1), is required for Lloviu virus (LLOV) entry, suggesting that receptor binding would not impose a barrier to LLOV infection. PMID: 25310500
- Data suggest that in order for the ligand cholesterol to slide from one binding pocket to the other (from NPC2 to NPC1), cholesterol undergoes conformational change/isomerization to accommodate the bent transfer pathway between the 2 binding pockets. PMID: 25251378
- A novel NPC1 mutation causing the Niemann-Pick type C disease and segregating to a Greek island has been identified. PMID: 23701245
- twelve individuals were subsequently confirmed to be NP-C by DNA analysis of NPC1 and NPC2 genes, with the early infantile form, the late infantile form, the juvenile form, and the adult form PMID: 24915861
- elevated mitochondrial cholesterol levels in NPC1-depleted cells and in NPC2-depleted cells expressing mutant NPC2 that allows endosomal cholesterol trafficking to mitochondria were associated with increased expression of antioxidant response factor Nrf2 PMID: 24790103
- role of NPC1 in regulating intracellular cholesterol trafficking and atherosclerosis. PMID: 24296264
- These findings show that the AD-like phenotype of NPC model cells can be partly reverted by promoting a non-amyloidogenic processing of APP through the upregulation of GGA1 supporting its preventive role against AD PMID: 24866237
- The minor G allele frequency of the rs1788799 polymorphisms in NPC1 might be a protective factor while the rs3764650 polymorphisms of ABCA7 might not be related to sporadic Alzheimer's disease in the Han Chinese population. PMID: 24064683
- the NPC1 promoter methylation is a probable mechanism that can result in reduced/impaired NPC1 expression/activity and may thus contribute to progression of cardiovascular diseases. PMID: 23567849
- Case Report: loss of NPC1 function, with attendant changes in membrane cholesterol composition, does not significantly modify the insulin resistance phenotype, even in the context of severely impaired INSR function. PMID: 20521171
- an atomistic model is proposed of the transfer of cholesterol from NPC2 to NPC1(NTD) through the formation of an intermediate NPC1(NTD)-NPC2 complex PMID: 24001314
- there is an additional sterol-binding site on NPC1 PMID: 23521797
- Treatment of NPC1-null or NPC2-deficient cells with cyclodextrin was effective in reducing cholesterol storage as well as the endocytic accumulation of sialoglycoproteins, demonstrating a direct link between cholesterol storage and abnormal recycling. PMID: 23733943
- lack of Npc1 protein can alter the expression profile of selected transcripts as well as proteins, and APP overexpression influences cerebral pathology by enhancing changes triggered by Npc1 deficiency in the bigenic line. PMID: 23382922
- Neuron-only expression of NPC1 does not completely prevent neurodegeneration; the addition of astrocyte expression decreases the rate of decline. PMID: 22495346
- this is the first report, showing a role of NPC1 in platelet function and formation but further studies are needed to define how cholesterol storage interferes with these processes PMID: 23010472
- Characterization of novel chromosomal microdeletions at 18q11-q12 involving the NPC1 gene in two patients with Niemann-Pick type C disease. PMID: 23142039
显示更多
收起更多
-
相关疾病:Niemann-Pick disease C1 (NPC1)
-
亚细胞定位:Late endosome membrane; Multi-pass membrane protein. Lysosome membrane; Multi-pass membrane protein.
-
蛋白家族:Patched family
-
数据库链接:
HGNC: 7897
OMIM: 257220
KEGG: hsa:4864
STRING: 9606.ENSP00000269228
UniGene: Hs.464779
Most popular with customers
-
-
YWHAB Recombinant Monoclonal Antibody
Applications: ELISA, WB, IF, FC
Species Reactivity: Human, Mouse, Rat
-
Phospho-YAP1 (S127) Recombinant Monoclonal Antibody
Applications: ELISA, WB, IHC
Species Reactivity: Human
-
-
-
-
-