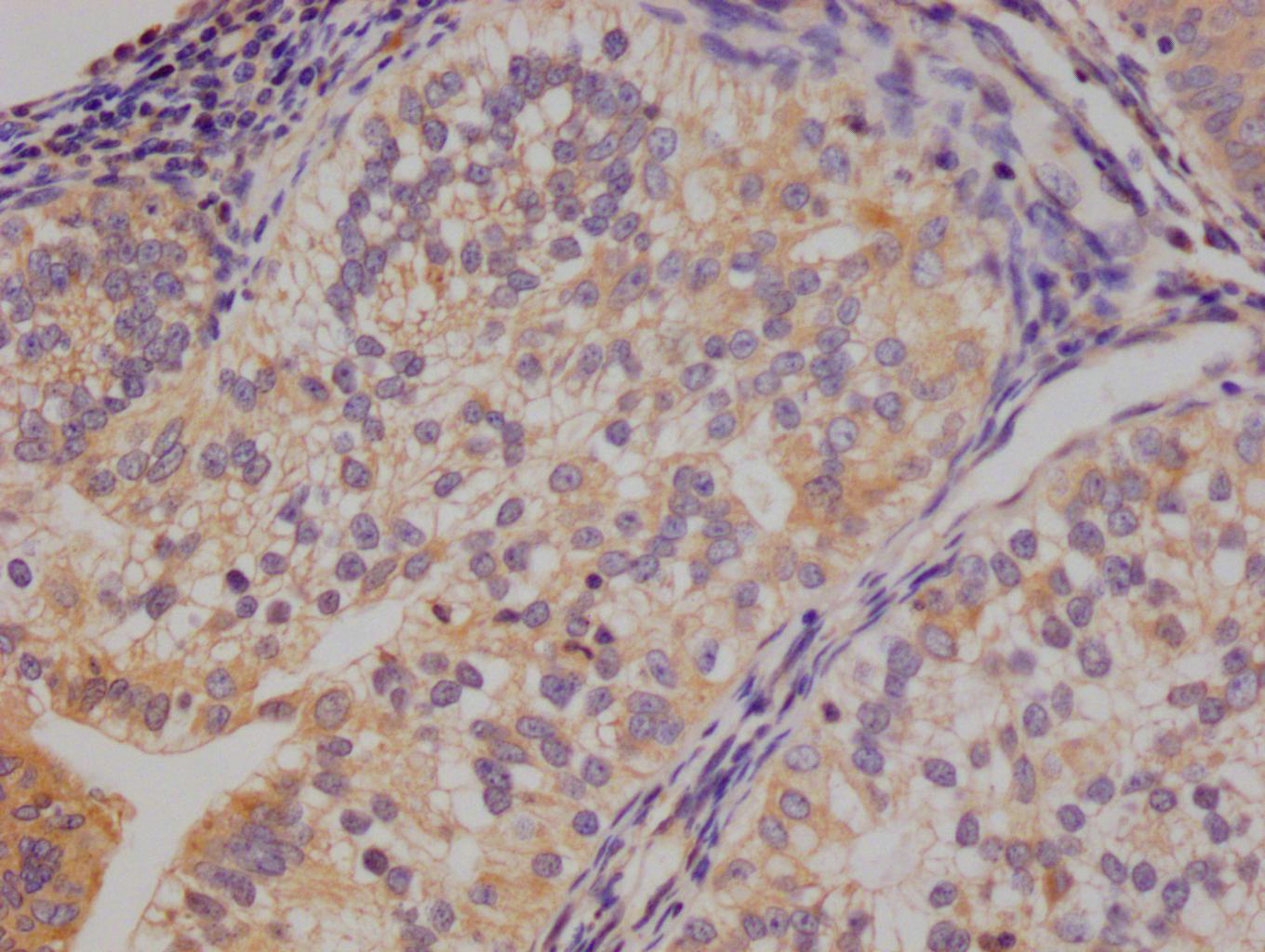
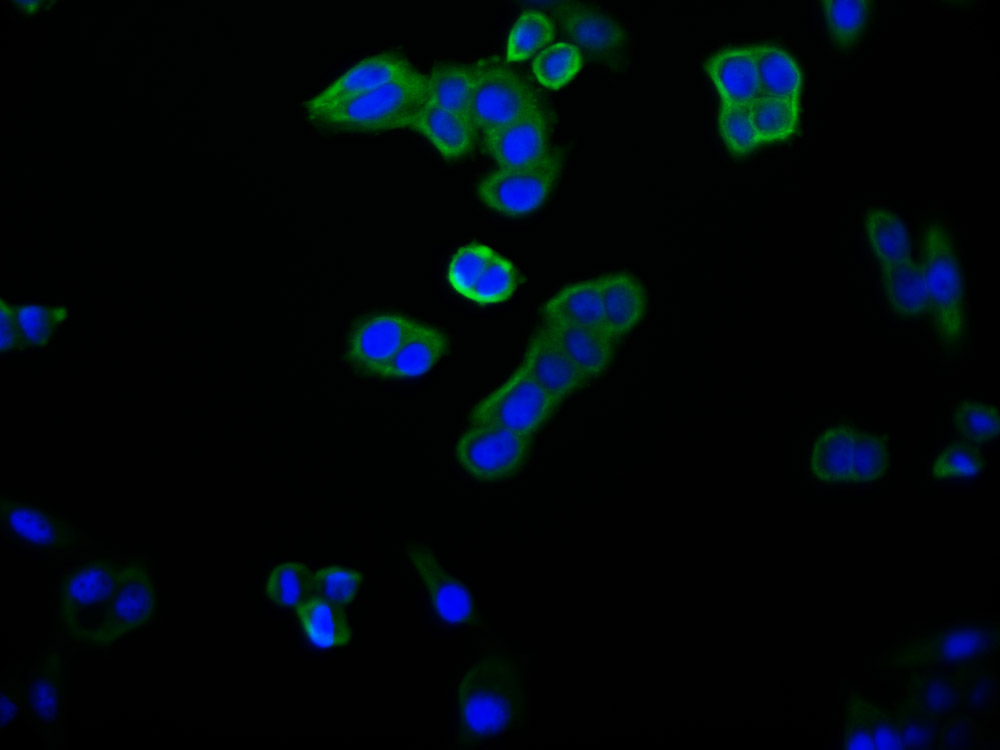
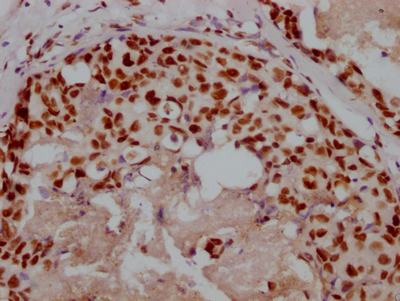
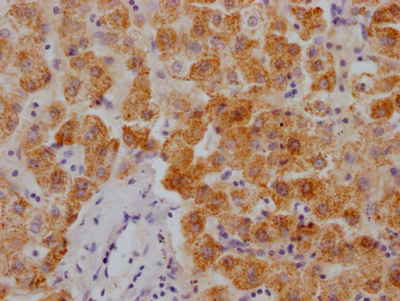
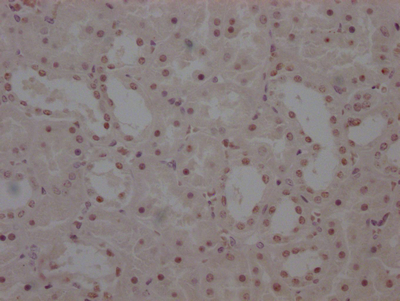
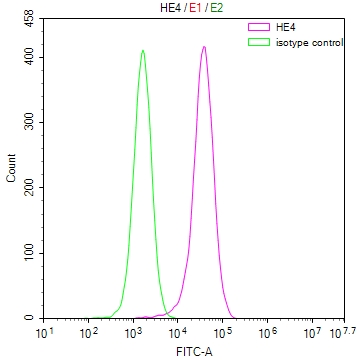
HUS1 Antibody
-
货号:CSB-PA010909GA01HU
-
规格:¥3,900
-
其他:
产品详情
-
Uniprot No.:O60921
-
基因名:
-
别名:Checkpoint protein HUS1 antibody; hHUS1 antibody; HUS1 (S. pombe) checkpoint homolog antibody; Hus1 antibody; HUS1 checkpoint homolog (S. pombe) antibody; HUS1 Checkpoint Protein antibody; HUS1 protein antibody; HUS1+ - like protein antibody; HUS1_HUMAN antibody; Hydroxyurea-sensitive 1, S. pombe, homolog of antibody
-
宿主:Rabbit
-
反应种属:Human,Mouse,Rat
-
免疫原:Human HUS1
-
免疫原种属:Homo sapiens (Human)
-
抗体亚型:IgG
-
纯化方式:Antigen Affinity purified
-
浓度:It differs from different batches. Please contact us to confirm it.
-
保存缓冲液:PBS with 0.1% Sodium Azide, 50% Glycerol, pH 7.3. -20°C, Avoid freeze / thaw cycles.
-
产品提供形式:Liquid
-
应用范围:ELISA,WB,IHC
-
Protocols:
-
储存条件:Upon receipt, store at -20°C or -80°C. Avoid repeated freeze.
-
货期:Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time maybe differs from different purchasing way or location, please kindly consult your local distributors for specific delivery time.
相关产品
靶点详情
-
功能:Component of the 9-1-1 cell-cycle checkpoint response complex that plays a major role in DNA repair. The 9-1-1 complex is recruited to DNA lesion upon damage by the RAD17-replication factor C (RFC) clamp loader complex. Acts then as a sliding clamp platform on DNA for several proteins involved in long-patch base excision repair (LP-BER). The 9-1-1 complex stimulates DNA polymerase beta (POLB) activity by increasing its affinity for the 3'-OH end of the primer-template and stabilizes POLB to those sites where LP-BER proceeds; endonuclease FEN1 cleavage activity on substrates with double, nick, or gap flaps of distinct sequences and lengths; and DNA ligase I (LIG1) on long-patch base excision repair substrates. The 9-1-1 complex is necessary for the recruitment of RHNO1 to sites of double-stranded breaks (DSB) occurring during the S phase.
-
基因功能参考文献:
- These results suggest that p21(Waf1/CIP1) and Hus1 play crucial roles in the generation and transmission of bystander damage signals after low-dose alpha-particle irradiation. PMID: 26600172
- adenine glycosylase activity, mismatch recognition properties and interaction with protein partners of MUTYH and 5 MAP variants were examined; P502L and R520Q had reduced affinity for PCNA; only Q324H was found to have reduced affinity for Hus1 PMID: 26377631
- Intramolecular binding of the rad9 C-terminus in the checkpoint clamp Rad9-Hus1-Rad1 is closely linked with its DNA binding. PMID: 26088138
- these pockets were not required for 9-1-1 chromatin localization or ATR-mediated CHK1 activation but were necessary for interactions between HUS1 and its binding partner MYH PMID: 25911100
- Expression of cell cycle regulatory factors hus1, gadd45a, rb1, cdkn2a and mre11a correlates with expression of clock gene per2 in human colorectal carcinoma tissue. PMID: 24062075
- HUS1 polymorphisms act as risk breast cancer modifiers in the group of non-carriers of BRCA1/2 mutations. PMID: 22926736
- Data show models for the ternary PCNA/FEN1/DNA and Rad9-Rad1-Hus1 (9-1-1 complex)/FEN1/DNA assemblies. PMID: 22586102
- The HUS1 is loaded to damaged sites where it serves as a platform for the selective recruitment of checkpoint and repair proteins. PMID: 21978893
- The HUS1 protein interacts with casein kinase 2. PMID: 20545769
- 9-1-1 complex is a component of the mismatch repair involved in MNNG-induced damage response. PMID: 20188637
- Rad9-Rad1-Hus1 complex enhances in vitro activity of 8-oxoguanine DNA glycosylase. PMID: 19615952
- downregulation by specific antisense oligonucleotides sensitizes human lung carcinoma cells to treatment with the DNA-damaging agent cisplatin PMID: 11920544
- Rad9, Hus1, and Rad1 heterotrimeric complex chromatin binding is a proximal event in the checkpoint signaling cascade PMID: 12228248
- HUS1 is an unstable protein that is actively degraded via the ubiquitin-proteasome pathway. Its expression can be stabilized by treating cells with proteasome-specific inhibitors. PMID: 15122316
- The human Rad9/Rad1/Hus1 complex interacts with and stimulates DNA polymerase beta activity. PMID: 15314187
- complex with rad1 and rad9 is a damage-specific activator of flap endonuclease 1 PMID: 15556996
- The long-patch base excision machinery is an important target of the Rad9-Rad1-Hus1 complex, thus enhancing the quality control of DNA. PMID: 15871698
- PCNA and the Rad9/Rad1/Hus1 complex can independently bind and activate Fen1; acetylation of Fen1 by p300-HAT abolished the stimulatory effect of the complex but not that of PCNA, suggesting a possible mechanism of regulation of this repair pathway PMID: 16216273
- human DNA ligase I is stimulated by the Rad9-rad1-Hus1 checkpoint complex PMID: 16731526
- These data provide in vivo evidence that the human 9-1-1 complex participates in DNA repair in addition to its previously described role in DNA damage sensing. PMID: 16814252
- Human NEIL1 DNA glycosylase activity is significantly stimulated by Hus1 and by the Rad9/Rad1/Hus1 heterotrimer. PMID: 17395641
- we report successful tri-cistronic cloning, overexpression and purification of a three-protein complex of Rad9, Rad1 and Hus1 using a single hexa-histidine tag. PMID: 17493829
- Jab1 physically associates with the 9-1-1 complex (Rad1-Rad9-Hus1) PMID: 17583730
- Human thymine DNA glycosylase activity is significantly stimulated by hHus1, hRad1, hRad9 separately, and by the 9-1-1 complex. PMID: 17855402
- Hus1 levels correlated significantly with the clinicopathologic factors of bad prognosis in epithelial ovarian tumors PMID: 18156970
- Loss of HUS1 sensitizes cells to etopside-induced apoptosis by regulating BH3-only proteins. PMID: 18794804
- The DNA binding domain (DBD) within the hLigI catalytic fragment interacts with both PCNA and the heterotrimeric cell-cycle checkpoint clamp, hRad9-hRad1-hHus1 (9-1-1). PMID: 19523882
- The interdomain connecting loops (IDC loop) of hRad9, hHus1, and hRad1 are largely divergent and unique structural features of the 9-1-1 complex that are proposed to contribute to DNA damage recognition. PMID: 19535328
- Functional study of the yeast homolog. PMID: 9524127
显示更多
收起更多
-
亚细胞定位:Nucleus. Cytoplasm, cytosol.
-
蛋白家族:HUS1 family
-
组织特异性:Ubiquitous.
-
数据库链接:
HGNC: 5309
OMIM: 603760
KEGG: hsa:3364
STRING: 9606.ENSP00000258774
UniGene: Hs.152983
Most popular with customers
-
-
YWHAB Recombinant Monoclonal Antibody
Applications: ELISA, WB, IF, FC
Species Reactivity: Human, Mouse, Rat
-
Phospho-YAP1 (S127) Recombinant Monoclonal Antibody
Applications: ELISA, WB, IHC
Species Reactivity: Human
-
-
-
-
-