HSPA1A Antibody
-
货号:CSB-PA10899A0Rb
-
规格:¥440
-
促销:
-
图片:
-
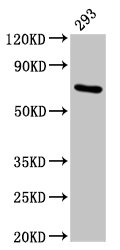
Western Blot
Positive WB detected in: 293 whole cell lysate
All lanes: HSPA1A antibody at 4.9µg/ml
Secondary
Goat polyclonal to rabbit IgG at 1/50000 dilution
Predicted band size: 71, 64 kDa
Observed band size: 71 kDa -
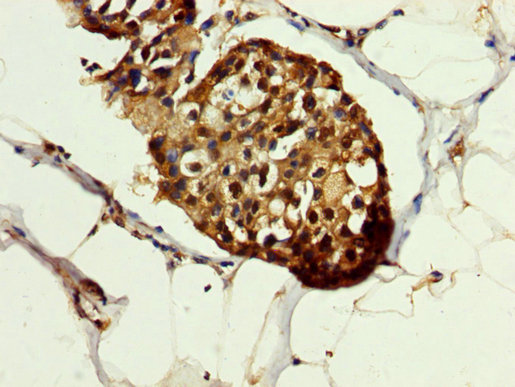
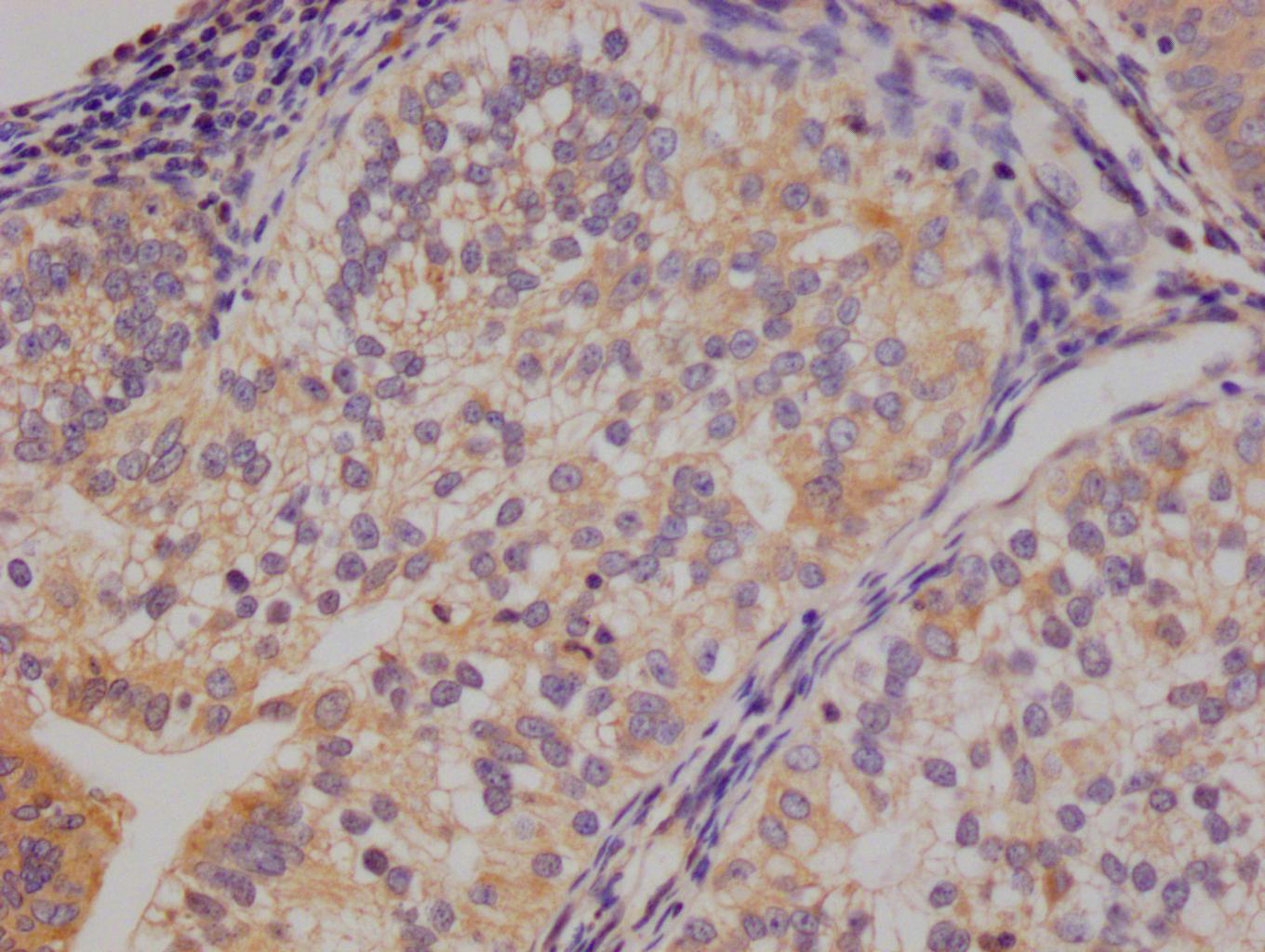
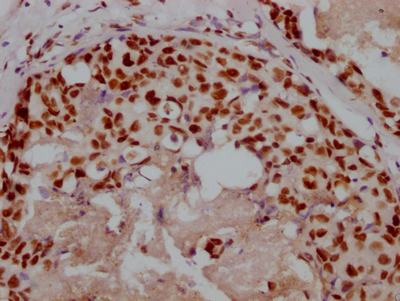
IHC image of CSB-PA10899A0Rb diluted at 1:200 and staining in paraffin-embedded human breast cancer performed on a Leica BondTM system. After dewaxing and hydration, antigen retrieval was mediated by high pressure in a citrate buffer (pH 6.0). Section was blocked with 10% normal goat serum 30min at RT. Then primary antibody (1% BSA) was incubated at 4°C overnight. The primary is detected by a biotinylated secondary antibody and visualized using an HRP conjugated SP system.
-
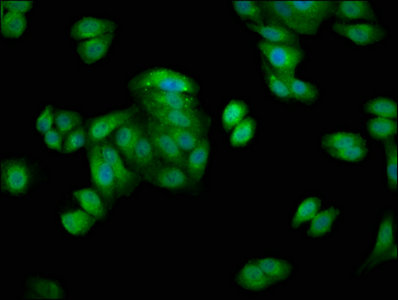
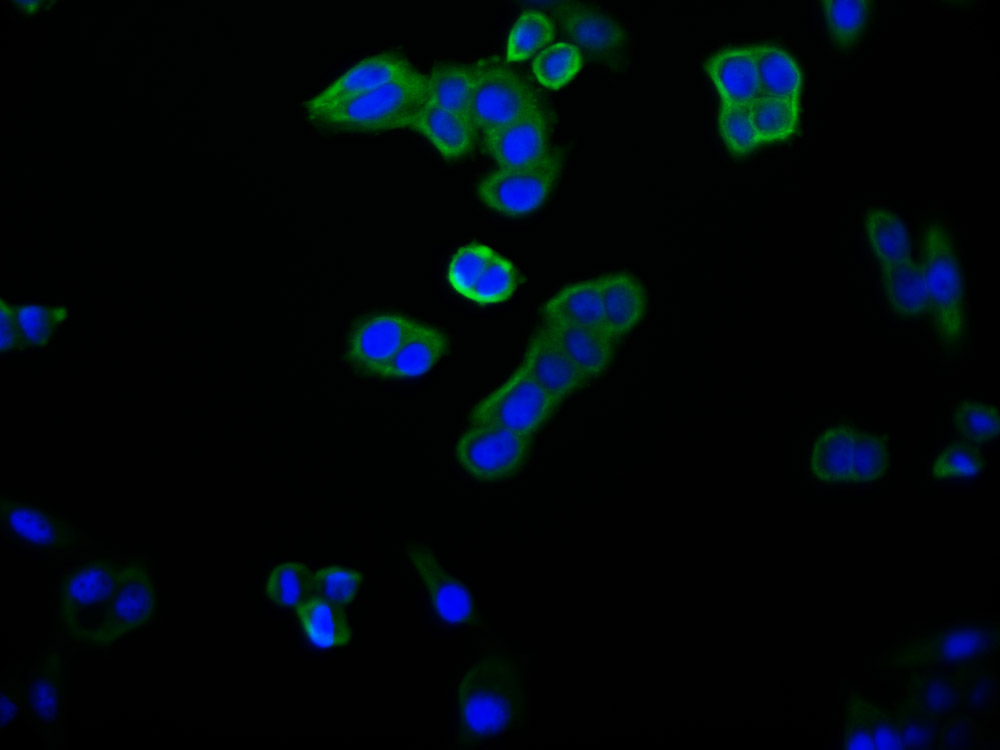
Immunofluorescence staining of HepG2 cells with CSB-PA10899A0Rb at 1:66, counter-stained with DAPI. The cells were fixed in 4% formaldehyde, permeabilized using 0.2% Triton X-100 and blocked in 10% normal Goat Serum. The cells were then incubated with the antibody overnight at 4°C. The secondary antibody was Alexa Fluor 488-congugated AffiniPure Goat Anti-Rabbit IgG(H+L).
-
-
其他:
产品详情
-
产品名称:Rabbit anti-Homo sapiens (Human) HSPA1A Polyclonal antibody
-
Uniprot No.:P0DMV8
-
基因名:HSPA1A
-
别名:HSPA1A antibody; HSP72 antibody; HSPA1 antibody; HSX70Heat shock 70 kDa protein 1A antibody; Heat shock 70 kDa protein 1 antibody; HSP70-1 antibody; HSP70.1 antibody
-
宿主:Rabbit
-
反应种属:Human
-
免疫原:Recombinant Human Heat shock 70 kDa protein 1A protein (418-512AA)
-
免疫原种属:Homo sapiens (Human)
-
标记方式:Non-conjugated
本页面中的产品,HSPA1A Antibody (CSB-PA10899A0Rb),的标记方式是Non-conjugated。对于HSPA1A Antibody,我们还提供其他标记。见下表:
-
克隆类型:Polyclonal
-
抗体亚型:IgG
-
纯化方式:>95%, Protein G purified
-
浓度:It differs from different batches. Please contact us to confirm it.
-
保存缓冲液:Preservative: 0.03% Proclin 300
Constituents: 50% Glycerol, 0.01M PBS, pH 7.4 -
产品提供形式:Liquid
-
应用范围:ELISA, WB, IHC, IF
-
推荐稀释比:
Application Recommended Dilution WB 1:500-1:5000 IHC 1:200-1:500 IF 1:50-1:200 -
Protocols:
-
储存条件:Upon receipt, store at -20°C or -80°C. Avoid repeated freeze.
-
货期:Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time maybe differs from different purchasing way or location, please kindly consult your local distributors for specific delivery time.
相关产品
靶点详情
-
功能:Molecular chaperone implicated in a wide variety of cellular processes, including protection of the proteome from stress, folding and transport of newly synthesized polypeptides, activation of proteolysis of misfolded proteins and the formation and dissociation of protein complexes. Plays a pivotal role in the protein quality control system, ensuring the correct folding of proteins, the re-folding of misfolded proteins and controlling the targeting of proteins for subsequent degradation. This is achieved through cycles of ATP binding, ATP hydrolysis and ADP release, mediated by co-chaperones. The co-chaperones have been shown to not only regulate different steps of the ATPase cycle, but they also have an individual specificity such that one co-chaperone may promote folding of a substrate while another may promote degradation. The affinity for polypeptides is regulated by its nucleotide bound state. In the ATP-bound form, it has a low affinity for substrate proteins. However, upon hydrolysis of the ATP to ADP, it undergoes a conformational change that increases its affinity for substrate proteins. It goes through repeated cycles of ATP hydrolysis and nucleotide exchange, which permits cycles of substrate binding and release. The co-chaperones are of three types: J-domain co-chaperones such as HSP40s (stimulate ATPase hydrolysis by HSP70), the nucleotide exchange factors (NEF) such as BAG1/2/3 (facilitate conversion of HSP70 from the ADP-bound to the ATP-bound state thereby promoting substrate release), and the TPR domain chaperones such as HOPX and STUB1. Maintains protein homeostasis during cellular stress through two opposing mechanisms: protein refolding and degradation. Its acetylation/deacetylation state determines whether it functions in protein refolding or protein degradation by controlling the competitive binding of co-chaperones HOPX and STUB1. During the early stress response, the acetylated form binds to HOPX which assists in chaperone-mediated protein refolding, thereafter, it is deacetylated and binds to ubiquitin ligase STUB1 that promotes ubiquitin-mediated protein degradation. Regulates centrosome integrity during mitosis, and is required for the maintenance of a functional mitotic centrosome that supports the assembly of a bipolar mitotic spindle. Enhances STUB1-mediated SMAD3 ubiquitination and degradation and facilitates STUB1-mediated inhibition of TGF-beta signaling. Essential for STUB1-mediated ubiquitination and degradation of FOXP3 in regulatory T-cells (Treg) during inflammation. Negatively regulates heat shock-induced HSF1 transcriptional activity during the attenuation and recovery phase period of the heat shock response. Involved in the clearance of misfolded PRDM1/Blimp-1 proteins. Sequesters them in the cytoplasm and promotes their association with SYNV1/HRD1, leading to proteasomal degradation.; (Microbial infection) In case of rotavirus A infection, serves as a post-attachment receptor for the virus to facilitate entry into the cell.
-
基因功能参考文献:
- binding of IL-5 to IL-5Ralpha receptors enhances angiogenic responses by stimulating the expression of HSP70-1 via the eNOS signaling pathway. PMID: 28317868
- Downregulation of HSPA1A impaired mesenchymal stem cell osteogenic and chondrogenic differentiation. PMID: 29323151
- HSPA1A overexpression promotes lipid accumulation in hepatocytes. PMID: 29631603
- Study demonstrates that HSP72 inhibits HDACi-induced apoptosis in Jurkat cell line. PMID: 29395577
- In conclusion, HSP70 modulates NF-kappaB activation in alveolar macrophages of TB patients, through inhibiting IkappaB-alpha phosphorylation or acting as a chaperon molecule to prevent NF-kappaB binding to the target genes by facilitating degradation. The upregulated HSP70 may suppress the release of pro-inflammatory cytokines during active pulmonary tuberculosis infection, and prevent overwhelming tissue damage. PMID: 28450725
- that HSPA6 and HSPA1A contribute to protection of differentiated human neuronal cells from cellular stress PMID: 29090408
- ultramarathon running caused a substantial increase in eHsp72 concentration, but probiotic + glutamine supplementation did not affect eHsp72 levels PMID: 28460195
- uHSP72 may be considered as a novel potential diagnostic biomarker for the early detection of Diabetic nephropathy (DN). Moreover, these data support the pivotal role of NLRP3 in the development and progression of DN PMID: 28631886
- The G allele of rs1008438G>T of HSPA1A may be a protective factor for cervical cancer among ethnic Han Chinese from Yunnan. PMID: 29188629
- measurable HSP72 was not associated with graft versus host disease following allogeneic hematopoietic cell transplantation PMID: 27020764
- studies demonstrated that ovarian cancer cells isolated from patients with type II tumors released high levels of immunosuppressive cytokines (i.e., interleukin 10 and transforming growth factor beta) and HspA1A in vitro. PMID: 26868087
- This study suggests that logotherapy affects the expression of cortisol, BDI, and pain scales in advanced cervical cancer patients, and that it does not affect the expression of HSP70. PMID: 27644267
- Data suggest that two putative NEF (nucleotide exchange factors) orthologs, GRPEL1 and GRPEL2, modulate function of mitochondrial HSP70 (mtHSP70); both GRPEL1 and GRPEL2 associate with mtHSP70 as hetero-oligomeric subcomplex and regulate mtHSP70 transport. (GRPEL = mitochondrial GrpE-like protein; HSP70 = heat-shock protein 70) PMID: 28848044
- High HSP72 expression is associated with Cluster Amplified Centrosomes in cancer. PMID: 28720575
- mRNA levels of HSP family members (HSP70B', HSP72, HSP40/DNAJ, and HSP20/CRYAB) are upregulated by the intracellular MMP3 overload. PMID: 27206651
- Data suggest that both ATP- and peptide-binding domains of HSPA1A can form complexes with an AU-rich element in VEGFA mRNA in vitro; only peptide-binding domain can recover cellular VEGFA mRNA in ribonucleoprotein immunoprecipitation; RNA-binding and mRNA-stabilizing functions of HSPA1A are independent of its protein chaperone cycle. (HSPA1A = heat shock 70 kDa protein 1A; VEGFA = vascular endothelial growth factor A) PMID: 28679534
- It has been demonstrated that HSPB8-BAG3-HSP70 ensures the functionality of stress granules and restores proteostasis by targeting defective ribosomal products for degradation. PMID: 27570075
- The rs2763979 locus of the HSP70 genes may be associated with susceptibility to noise-induced hearing loss (NIHL) in Chinese individuals, and other HSP70 genes may also be susceptibility genes for NIHL, but the results must be further replicated in additional independent sample sets. PMID: 28182740
- These results suggest that NFkappaB engaged with the kappaB motif on the promoter cooperates in Hsp70A1A activation under heat shock in human cells as part of a DNA-break repair complex including DNA-PK and PARP-1. PMID: 28099440
- Systematic proteomic identification of the heat shock proteins that interact with estrogen receptor alpha and biochemical characterization of the ERalpha-hsp70 interaction has been reported. PMID: 27483141
- epidermal Hsp70-1A contributes to the diversity of skin color by regulating the amount of melanin synthesized in melanocytes and modulating autophagic melanosome degradation in keratinocytes PMID: 27094592
- Extracellular Hsp72 immediately post-exercise decreased back to baseline levels by 1 h post-exercise, but cellular Hsp72 expression continued to rise and remained elevated 24 h post-exercise. These data suggest that in addition to the classic physiological biomarkers of exercise heat stress, using cellular Hsp72 as an indicator of lasting effects of stress into recovery may be most appropriate for determining long-term ef PMID: 26643874
- HSPA1A and HSPA8 have roles in parturition through stimulating immune inflammatory and estrogen response PMID: 28025138
- Data show that BAG2 Inhibits CHIP-Mediated HSP72 ubiquitination in aged cells. PMID: 28042827
- indicates increased expression levels of heat shock proteins 90 and 70 and glucose related protein 78 levels in medullary thyroid carcinoma PMID: 28038712
- The cardioprotective effect of 40-60 g/d of alcohol consumption could be due in part, to increased intracellular HSPA1A, a potent anti-inflammatory protein. Excessive intake of alcohol increases antibodies anti-Hsp60, stimulating proinflammatory cytokines. This fact may explain the mortality from cardiovascular disease in heavy drinkers. PMID: 26902796
- There is a direct correlation between plasma HSPA1A and PAI-1 levels in patients with diabetes, which is lost when they develop albuminuria. PMID: 26637413
- The present study revealed that salivary extracellular HSP70 significantly increased at 4 h after the 59 min of intense exercise in sedentary male subjects and correlated with resting salivary secretory immunoglobulin A (SIgA) levels at rest. PMID: 26608509
- HSP70-2 (+1267A/G) gene polymorphism was associated with Henoch-Schonlein purpura in children PMID: 26547206
- HSP72 blocks fibroblast activation and proliferation in renal fibrosis via targeting the STAT3 pathway and may serve as a novel therapeutic agent for chronic kidney disease regardless of the etiology PMID: 26851345
- Studies suggest that heat shock protein 72/70 (Hsp70 proteins) are beneficial to the patient in slowing the onset of neurodegenerative disorders. PMID: 26450908
- P53 could be used to distinguish early HCC from advanced hepatocellular carcinoma, but HSP70 cannot PMID: 26494212
- cytokines, not being influenced by HSP72 polymorphisms, cortisol, or illness severity. Gln may depress l/mHSP72 after LPS exposure and enhance them after HS induction, but it may not affect early induced HSP72 mRNA. PMID: 26550577
- heat acclimation reduces physiological strain, and the transcription of HSP72, but not HSP90alpha mRNA in acute normobaric hypoxia. PMID: 26205540
- Hsp72 (HSPA1A) prevents h-IAPP aggregation and toxicity. PMID: 26960140
- HSPA1A (rs1043618) is associated with a decreased risk of idiopathic pulmonary fibrosis in a Mexican population. PMID: 26496868
- Lysine methylation of HSPA1 differs between metastatic breast and ovarian carcinoma. PMID: 26448330
- Together, these results implicate HSP70-1A as a novel angiogenic regulator. PMID: 26657847
- The aim of this study was to demonstrate the effects of 6-week low-intensity training on changes in indicators of aerobic capacity and on HSPA1A, HSPB1, and LDHb expression in white blood cells in high level rowers. PMID: 26214432
- BDNF, APOE, and HSP70-1 genes, but not GRIN2B, might be associated with a risk of POAG occurrence in the Polish population PMID: 25893192
- Leukocyte Hsp72 mRNA was increased immediately after exercise following downhill running compared to flat running and in hot compared to temperate conditions. PMID: 25722377
- Equal Hsp72 mRNA increases occurring from consistent, reduced, or increased endogenous strain following short-term heat acclimation and long-term heat acclimation suggest that transcription occurs following attainment of sufficient endogenous criteria. PMID: 25943677
- higher levels of plasma Hsp70 and lower levels of plasma Hsp27 might be associated with an increased risk of COPD among coal workers. PMID: 25620081
- Serum HSPA1A levels correlate with disease status in rheumatoid arthritis. PMID: 25739548
- This is indicative of improved tolerance and ability to cope with the hypoxic insult, potentially mediated in part by increased basal reserves of HSP72. PMID: 25874231
- Data indicate that the smallest average tumor weight was present in the AdSurp-heat shock 70kDa protein (Hsp70)+CIK treatment group. PMID: 25473902
- Results report high resolution crystal structure of the substrate-bound human HSP70-substrate-bound domain and particularly the alpha and beta loops. PMID: 25058147
- HSP72 preserves renal function in I/R injury through its antiapoptotic effects, which act by suppressing mitochondrial Smac/Diablo release and preserving XIAP protein content. PMID: 25394481
- The results demonstrate a key role for inducible HSP70 in aiding the processing and hindering the accumulation of misfolded PMP22, which in turn alleviates proteotoxicity within the cells. PMID: 25694550
- Nek6 facilitates association of Hsp72 with the mitotic spindle, where it promotes stable K-fiber assembly through recruitment of the ch-TOG-TACC3 complex. PMID: 25940345
显示更多
收起更多
-
亚细胞定位:Cytoplasm. Nucleus. Cytoplasm, cytoskeleton, microtubule organizing center, centrosome. Secreted. Note=Localized in cytoplasmic mRNP granules containing untranslated mRNAs.
-
蛋白家族:Heat shock protein 70 family
-
数据库链接:
HGNC: 5232
OMIM: 140550
KEGG: hsa:3303
STRING: 9606.ENSP00000364802
UniGene: Hs.274402
Most popular with customers
-
-
YWHAB Recombinant Monoclonal Antibody
Applications: ELISA, WB, IF, FC
Species Reactivity: Human, Mouse, Rat
-
Phospho-YAP1 (S127) Recombinant Monoclonal Antibody
Applications: ELISA, WB, IHC
Species Reactivity: Human
-
-
-
-
-