ETF1 Antibody
-
货号:CSB-PA007840GA01HU
-
规格:¥3,900
-
其他:
产品详情
-
Uniprot No.:P62495
-
基因名:ETF1
-
别名:Cl1 protein antibody; D5S1995 antibody; ERF antibody; eRF1 antibody; ERF1_HUMAN antibody; ETF1 antibody; Eukaryotic peptide chain release factor subunit 1 antibody; Eukaryotic release factor 1 antibody; Eukaryotic translation termination factor 1 antibody; MGC111066 antibody; Polypeptide chain release factor 1 antibody; Protein Cl1 antibody; RF1 antibody; Sup45 (yeast omnipotent suppressor 45) homolog like 1 antibody; SUP45L1 antibody; TB3 1 antibody; TB3-1 antibody
-
宿主:Rabbit
-
反应种属:Human,Mouse,Rat
-
免疫原:Human ETF1
-
免疫原种属:Homo sapiens (Human)
-
抗体亚型:IgG
-
纯化方式:Antigen Affinity Purified
-
浓度:It differs from different batches. Please contact us to confirm it.
-
保存缓冲液:PBS with 0.1% Sodium Azide, 50% Glycerol, pH 7.3. -20°C, Avoid freeze / thaw cycles.
-
产品提供形式:Liquid
-
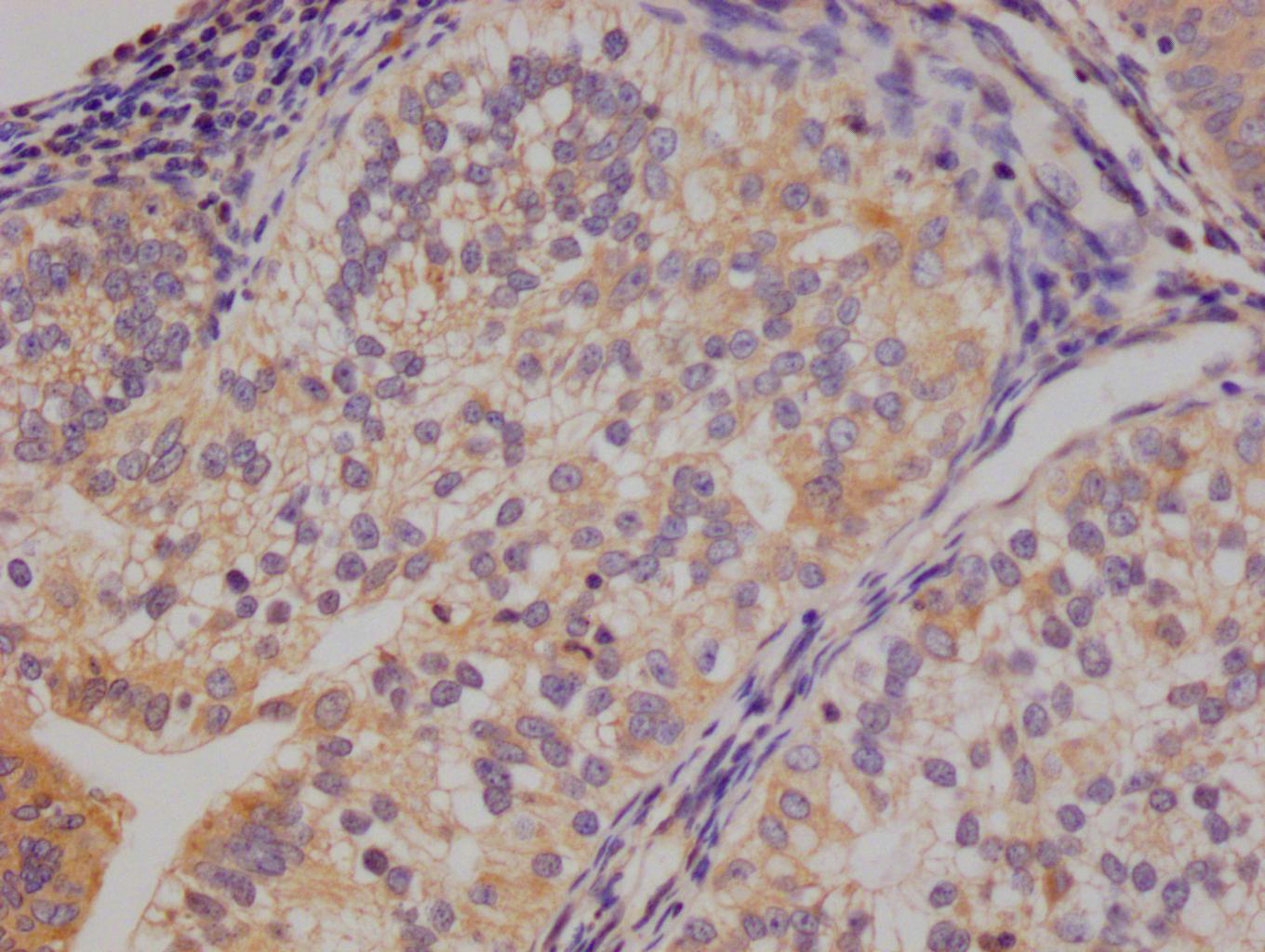
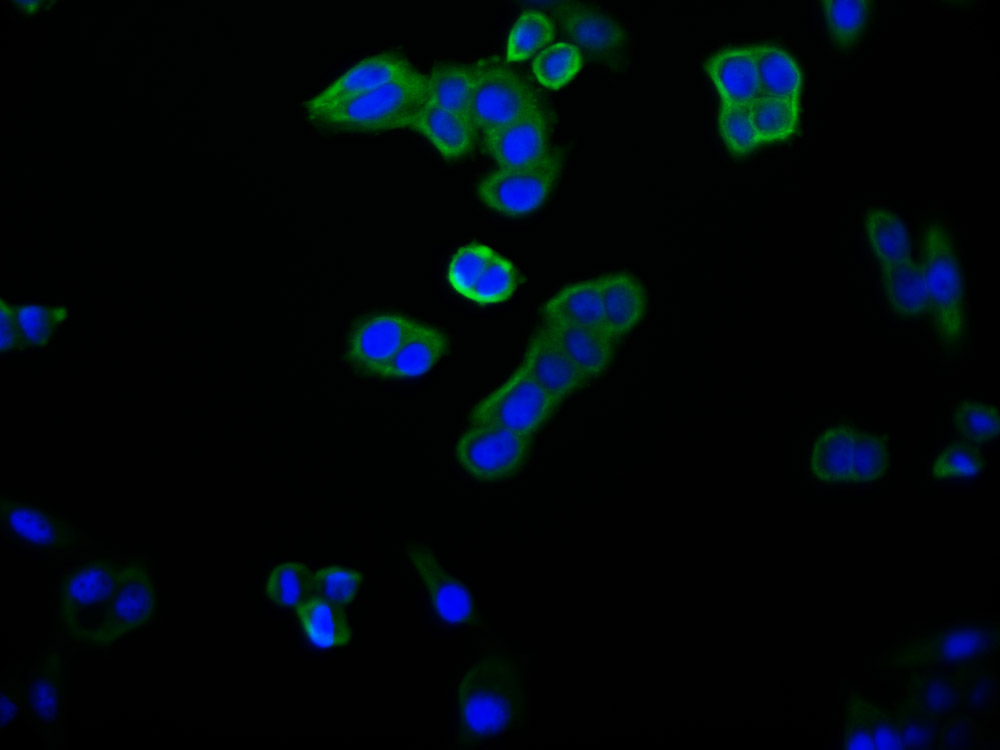
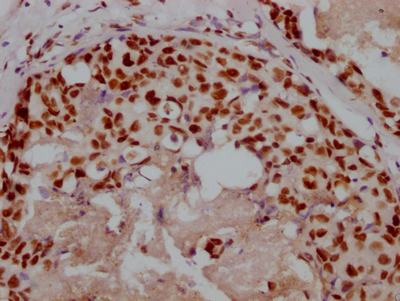
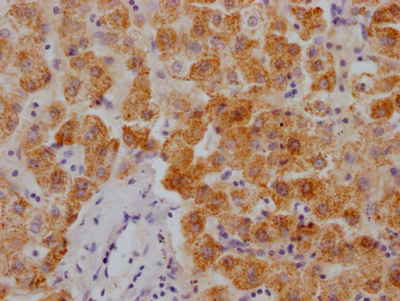
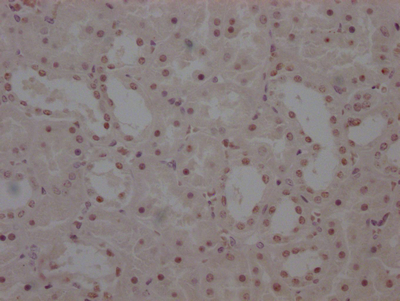
应用范围:ELISA,WB,IHC
-
Protocols:
-
储存条件:Upon receipt, store at -20°C or -80°C. Avoid repeated freeze.
-
货期:Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time maybe differs from different purchasing way or location, please kindly consult your local distributors for specific delivery time.
相关产品
靶点详情
-
功能:Directs the termination of nascent peptide synthesis (translation) in response to the termination codons UAA, UAG and UGA. Component of the transient SURF complex which recruits UPF1 to stalled ribosomes in the context of nonsense-mediated decay (NMD) of mRNAs containing premature stop codons. Required for SHFL-mediated translation termination which inhibits programmed ribosomal frameshifting (-1PRF) of mRNA from viruses and cellular genes.
-
基因功能参考文献:
- Report molecular dynamics free energy calculations on termination complexes, where relative eRF1 binding free energies to different cognate and near-cognate codons are evaluated. The simulations show a high and uniform discrimination against the near-cognate codons, that differ from the cognate ones by a single nucleotide, and reveal the structural mechanisms behind the precise decoding by eRF1. PMID: 29127299
- The GTS loop forms a switch that is key for the multiple codon recognition capability of eRF1. PMID: 26725946
- New information has been presented on architecture of the eRF1 binding site on mammalian ribosome at various translation termination steps and on conformational rearrangements induced by binding of the release factors. PMID: 26655225
- cryo-electron microscopy (cryo-EM) structures at 3.5-3.8 A resolution of ribosomal complexes containing eRF1 interacting with each of the three stop codons in the A-site PMID: 26245381
- We characterized a region of the eRF1 N-terminal domain, the P1 pocket, that we had previously shown to be involved in termination efficiency. We identified two residues, arginine 65 and lysine 109, as critical for recognition of the three stop codons. PMID: 25735746
- C4 lysyl hydroxylation of eRF1 is required for optimal translational termination PMID: 24486019
- The role of the 41 invariant and conserved N-domain residues in stop codon decoding by human eRF1 was determined. PMID: 23435318
- This work provides mechanistic insight into the coordination between GTP hydrolysis by eRF3 and subsequent peptide release by eRF1. PMID: 23091004
- s propose that structural variability in the GTS loop may underline the switching between omnipotency and unipotency of eRF1, implying the direct access of the GTS loop to the stop codon. PMID: 22383581
- The NMR data show that the N-domain of human eRF1 exists in two conformational states. PMID: 22517631
- Molecular modeling of eRF1 in the 80S termination complex showed that eRF1 fragments neighboring guanines and adenines of stop signals are compatible with different N domain conformations of eRF1. PMID: 21602268
- By molecular modeling, the eRF1 molecule can be fitted to the A site proximal to the P-site-bound tRNA and to a stop codon in mRNA via a large conformational change to one of its three domains. PMID: 20688868
- Data show that depleting eRF1 increased the Gag-Pol to Gag ratio in cells infected with replication-competent virus. PMID: 20418372
- bacterial polypeptide release factor RF2 is structurally distinct from eukaryotic eRF1 PMID: 11779511
- The invariant uridine of stop codons contacts the conserved NIKSR loop in the ribosome PMID: 12356746
- codon dependence of human eRF1 binding to the mRNA-ribosome complex PMID: 12909007
- the intracellular concentration of the eukaryotic release factor 1 (eRF1) is a critical parameter influencing the efficiency of amino acid incorporation by nonsense suppression PMID: 15716307
- Glu55 and Tyr125 residues in the N domain of eRF1 are important for eRF1's decoding function. PMID: 16282590
- we describe a novel complex that contains the NMD factors SMG-1 and Upf1, and the translation termination release factors eRF1 and eRF3 (SURF). PMID: 16452507
- Results shows eRF1 promotes GTP binding by eRF3. PMID: 16797113
- Interface of the interaction of the middle domain of human translation termination factor eRF1 with eukaryotic ribosomes PMID: 19140327
- Molecular dynamics simulations show that there is no structural effect on the free RF1 release factor caused by methylation of glutamine185, suggesting that its role is intimately associated with the ribosome environment. PMID: 19265422
显示更多
收起更多
-
亚细胞定位:Cytoplasm.
-
蛋白家族:Eukaryotic release factor 1 family
-
数据库链接:
HGNC: 3477
OMIM: 600285
KEGG: hsa:2107
STRING: 9606.ENSP00000353741
UniGene: Hs.483494
Most popular with customers
-
-
YWHAB Recombinant Monoclonal Antibody
Applications: ELISA, WB, IF, FC
Species Reactivity: Human, Mouse, Rat
-
Phospho-YAP1 (S127) Recombinant Monoclonal Antibody
Applications: ELISA, WB, IHC
Species Reactivity: Human
-
-
-
-
-