DEFA5 Antibody
-
货号:CSB-PA245088
-
规格:¥1100
-
图片:
-
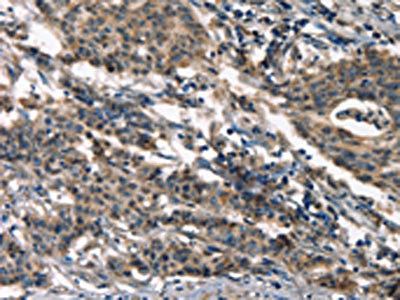
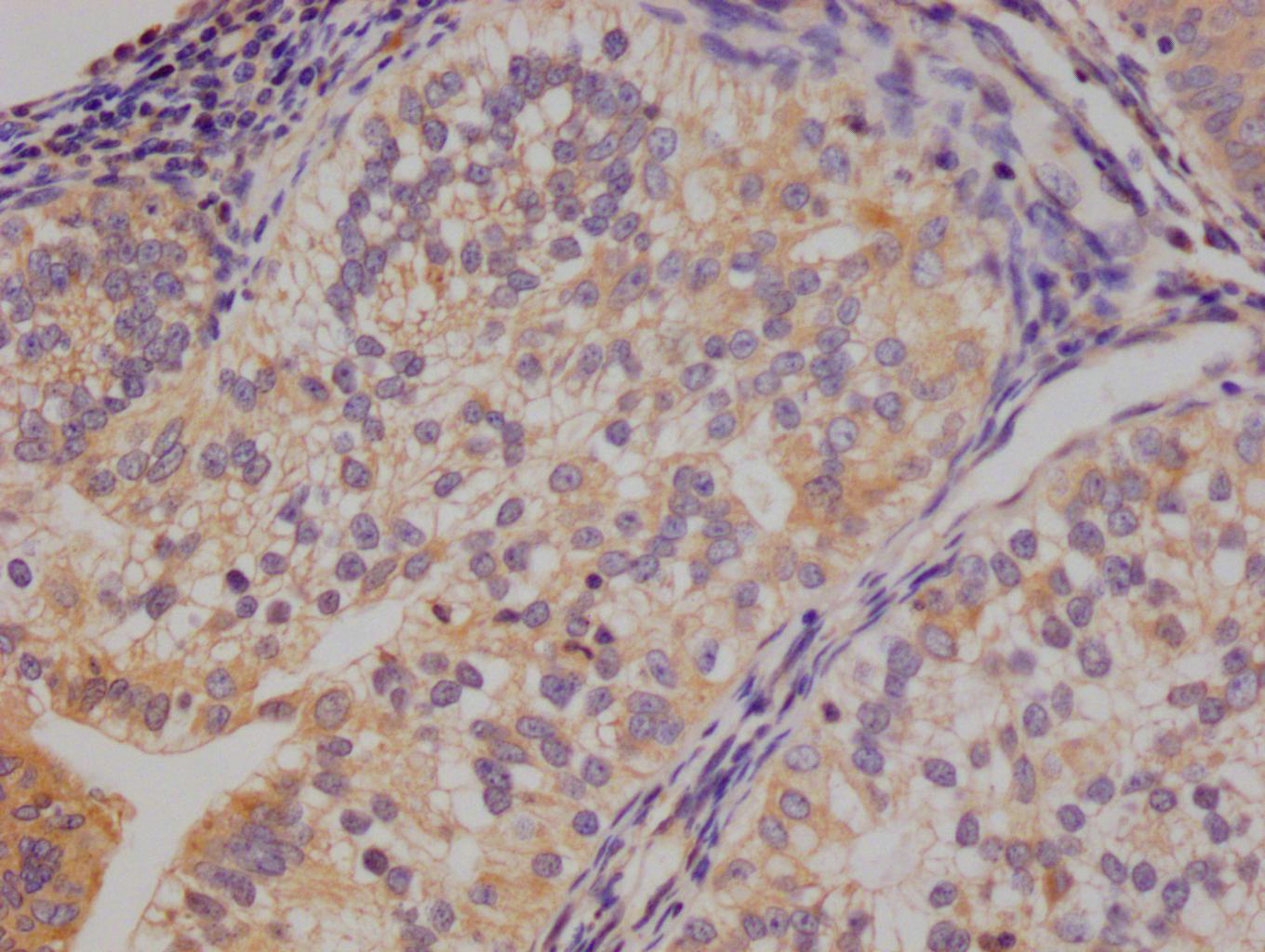
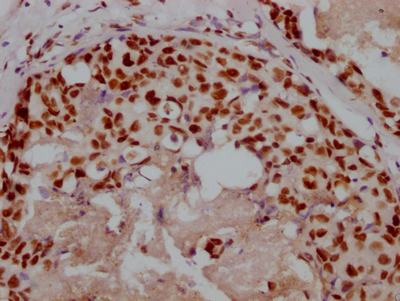
The image on the left is immunohistochemistry of paraffin-embedded Human gastic cancer tissue using CSB-PA245088(DEFA5 Antibody) at dilution 1/15, on the right is treated with synthetic peptide. (Original magnification: ×200)
-
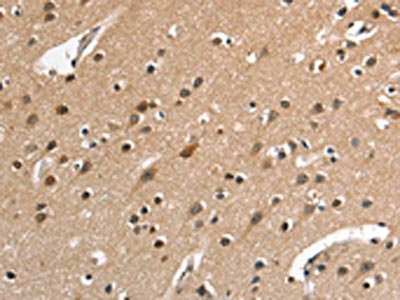
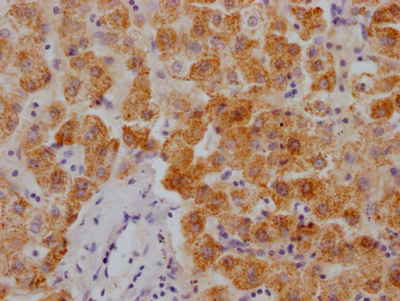
The image on the left is immunohistochemistry of paraffin-embedded Human brain tissue using CSB-PA245088(DEFA5 Antibody) at dilution 1/15, on the right is treated with synthetic peptide. (Original magnification: ×200)
-
-
其他:
产品详情
-
Uniprot No.:Q01523
-
基因名:DEFA5
-
别名:alpha 5 antibody; DEF5 antibody; DEF5_HUMAN antibody; Defa antibody; Defa29 antibody; DEFA5 antibody; Defcr29 antibody; Defcr5 antibody; Defensin 5 antibody; Defensin alpha 5 antibody; Defensin alpha 5 Paneth cell specific antibody; Defensin alpha 5 preproprotein antibody; Defensin antibody; Enteric defensin antibody; HD 5 antibody; HD5 antibody; HD5(20-94) antibody; HD5(63-94) antibody; MGC129728 antibody; RD 5 antibody
-
宿主:Rabbit
-
反应种属:Human
-
免疫原:Synthetic peptide of Human DEFA5
-
免疫原种属:Homo sapiens (Human)
-
标记方式:Non-conjugated
-
抗体亚型:IgG
-
纯化方式:Antigen affinity purification
-
浓度:It differs from different batches. Please contact us to confirm it.
-
保存缓冲液:-20°C, pH7.4 PBS, 0.05% NaN3, 40% Glycerol
-
产品提供形式:Liquid
-
应用范围:ELISA,IHC
-
推荐稀释比:
Application Recommended Dilution ELISA 1:1000-1:2000 IHC 1:15-1:50 -
Protocols:
-
储存条件:Upon receipt, store at -20°C or -80°C. Avoid repeated freeze.
-
货期:Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time maybe differs from different purchasing way or location, please kindly consult your local distributors for specific delivery time.
相关产品
靶点详情
-
功能:Has antimicrobial activity against Gram-negative and Gram-positive bacteria. Defensins are thought to kill microbes by permeabilizing their plasma membrane. All DEFA5 peptides exert antimicrobial activities, but their potency is affected by peptide processing.
-
基因功能参考文献:
- The binding mechanisms of HD5 on lipopolysaccharide in comparison to a bare DMPC lipid membrane was studied using molecular dynamics (MD) simulations. PMID: 30187138
- Bifidobacterium infantis NLS super strain decreases Paneth cell counts and expression of alpha-defensin-5 in celiac disease. PMID: 27636409
- Human alpha-Defensin-5 is a potential candidate biomarker to molecularly differentiate Crohn's colitis from ulcerative colitis, to our knowledge. These data give us both a potential diagnostic marker in Human alpha-Defensin-5 and insight to develop future mechanistic studies to better understand crypt biology in Crohn's colitis. PMID: 28817680
- Different dynamics and pathway of disulfide bonds reduction has been demonstrated for two human defensins, HD5 and HBD-3. PMID: 28106297
- These data support capsid stabilization and redirection to the lysosome during infection as a general antiviral mechanism of alpha-defensins against nonenveloped viruses. PMID: 28119475
- Human adenovirus 3 produces large amounts of penton-dodecahedra (PtDd), virus-like particles, during replication, which also function in blocking of HD5. PMID: 28077642
- no biologic differences between wild-type HD5 and its variants were observed on HD5 antibacterial activity against Escherichia coli PMID: 27160989
- a moderately active linear peptide derived from the alpha-defensin HD5 can be engineered to enhance antimicrobial activity almost comparable to the native peptide. PMID: 26206286
- The study used ion mobility coupled to mass spectrometry to track the structural changes in HD-5 upon disulfide bond reduction. PMID: 25970658
- The study reports the screening of human defensin 5 against the Keio Collection of E. coli strains and shows how this important hostdefense peptide kills bacteria and how bacteria protect themselves against the attack from the human host. PMID: 25430675
- s show that although NOD2 by itself can slightly up-regulate expression of HD5 and HD6, it can strongly down-regulates their expression during differentiation of the Paneth cell lineage mainly by inhibiting activation of the MAPK pathway. PMID: 25433720
- HD5ox, at least in part, exerts its antibacterial activity against E. coli and other Gram-negative microbes in the cytoplasm. PMID: 25664683
- These data support a model in which HD5 prevents furin from accessing human papillomavirus 16 L2 by occluding the furin cleavage site via direct binding to the viral capsid. PMID: 25540379
- Enteric alpha-defensin HD5 and beta-defensin hBD2 shared similar toxin-unfolding effects with HNP1, albeit to different degrees. PMID: 25517613
- our results highlight the potential contribution of altered alpha-defensin HD-5 expression in the formation of a viral/tumour-permissive environment in high-risk HPV infection and progression to cancer of the uterine cervix PMID: 25196670
- DEFA5 displays antimicrobial activity against E. coli, E. aerogenes, B. cereus, and S. aureus. PMID: 15616305
- Data indicate that cellular effects of defensin 5 involve interactions with the extra-cellular domain of tumor necrosis Factor 1. PMID: 24681099
- Anti-HIV activity of human defensin 5 in primary CD4+ T cells under serum-deprived conditions is a consequence of defensin-mediated cytotoxicity. PMID: 24086683
- This study considers the redox properties of HD-5 and reports that the reduced form, HD-5 red, is a zinc-ion chelator. PMID: 23841778
- HD5 neutralizes JC polyomavirus infection by stabilizing the viral capsid and disrupting virus trafficking. PMID: 24198413
- human alpha defensin 5 increases LGR stem cell migration into wound beds, leading to enhanced healing, bacterial reduction, and hair production through the augmentation of key Wnt and wound healing transcripts. PMID: 24165598
- E-cadherin expression in squamous cells is reduced by HD-5 PMID: 23958301
- Subnanometer resolution cryo-electron microscopy structures of HD5 complexed with both neutralization-sensitive and -resistant human adenovirus chimeras, were determined. PMID: 23620768
- study demonstrate that binding of integrin alphavbeta5 and alpha defensin 5 have opposite effects on the elastic response of adenovirus type 35, revealing a direct link between virus-host interactions and the mechanical properties of the capsid PMID: 23269786
- structural analysis of human enteric alpha-defensin HD5 shows a crucial role for hydrophobicity at the dimer interface PMID: 22573326
- Several arginine residues and tyrosine 27 are important for HD-5 antibacterial activity. PMID: 22354633
- Defensins 5 and 6 enhance HIV-1 infectivity through promoting HIV attachment. PMID: 21672195
- The hydrophobicity of regions 2, 9 & 10 was higher than in other regions. It was difficult for the HD molecule to have many conformations because of its 3 S-S bridges; however, it responds to various external factors by restricting conformation. PMID: 21873175
- The concentrations of human defensin 5, HBD1, and HBD2 in vulvovaginal candidiasis patients were higher than in controls. PMID: 19080508
- Statistical analysis of gene expression showed a distinction between regions 1 and 2 of the small intestin for HD5. PMID: 21125297
- HalphaD-5 and HbetaD-2 may protect fallopian tubes during microbial infection. PMID: 20629326
- To respond to LPS stimulation, human primary endocervical epithelial cells may function in the mucosal immune defense through TLR4 activation and HD5 secretion. PMID: 20819643
- This study demonstrated the multifunctional roles of the activation process in human defensin-5. PMID: 20375624
- The s provide direct evidence that human alpha-defensins block adenovirus infection by preventing uncoating during cell entry. PMID: 20130047
- Data show that DEFA5-expressing mice had striking losses of segmented filamentous bacteria and fewer interleukin 17 (IL-17)-producing lamina propria T cells. PMID: 19855381
- By acting as a prodefensin convertase in human Paneth cells, trypsin is involved in the regulation of innate immunity in the small intestine. PMID: 12021776
- Defensin 5 transgenic mice were resistant to oral challenge with virulent Salmonella typhimurium; support for a critical in vivo role of epithelial-derived defensins in mammalian host defence PMID: 12660734
- Crystal structure of HD-5. PMID: 17088326
- HD-5 binds to the cell membrane of intestinal epithelial cells and induces secretion of the chemokine interleukin (IL)-8 PMID: 17250830
- The persistence of HD5-chymotrypsinogen-trypsin complex in Crohn disease may be attributable to increased luminal levels of proteinase inhibitors such as alpha1-anti-trypsin. PMID: 18258845
- Single nucleotide polymorphism in defensin alpha 5 Paneth cell associated with inflammatory bowel diseases in caucasoid population. PMID: 18394979
- Clostridium difficile toxin B interacts with high affinity with HD5 which may provide a defense mechanism against clostridial glucosylating cytotoxins. PMID: 18435932
- the primary function of the conserved salt bridge in HD5 is to ensure correct processing of proHD5 and subsequent stabilization of mature alpha-defensin in vivo PMID: 18499668
- HBD-2 and HD-5 may be involved in defending against invasion by BV-related microorganisms and the decrease in IL-4 concentration may increase susceptibility to bacterial vaginosis PMID: 18635180
- Human enteric defensin (HD)-5 and HD-6 were detected in Paneth cells, which are observed in the gastric metaplastic mucosa as well as small intestinal epithelia. Less H. pylori was observed in the intestinal metaplasia with HD-5 expressing Paneth cells. PMID: 19250512
- Self-association and multivalent binding may play integral roles in the ability of alpha-defensin 5 (HD5) to protect against infections caused by viruses and other infectious agents. PMID: 19542459
- Results report on the antibacterial properties and cellular interaction of Human Defensin 5 as a function of its positive charge and hydrophobicity. PMID: 19589339
显示更多
收起更多
-
亚细胞定位:Secreted. Cytoplasmic vesicle, secretory vesicle. Note=Peptides HD5(20-94), HD5(23-94) and HD5(29-94) are found within tissues, HD5(20-94) being the predominant intracellular form. Peptides HD5(56-94) and HD5(63-94) are found in the extracellular milieu, HD5(63-94) being the most abundant form.
-
蛋白家族:Alpha-defensin family
-
组织特异性:Paneth cells of the small intestine (at protein level).
-
数据库链接:
HGNC: 2764
OMIM: 600472
KEGG: hsa:1670
STRING: 9606.ENSP00000329890
UniGene: Hs.655233
Most popular with customers
-
-
YWHAB Recombinant Monoclonal Antibody
Applications: ELISA, WB, IF, FC
Species Reactivity: Human, Mouse, Rat
-
Phospho-YAP1 (S127) Recombinant Monoclonal Antibody
Applications: ELISA, WB, IHC
Species Reactivity: Human
-
-
-
-
-