CCS Antibody
-
货号:CSB-PA297584
-
规格:¥2024
-
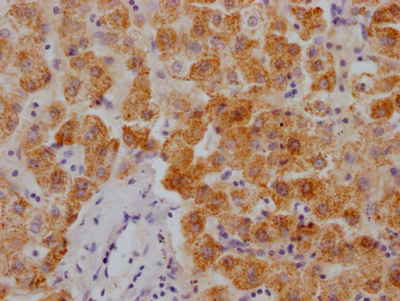
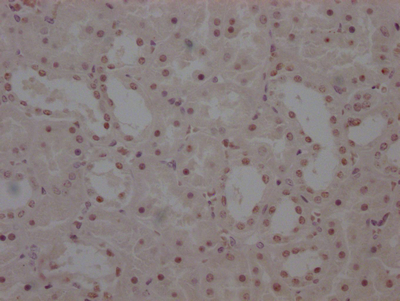
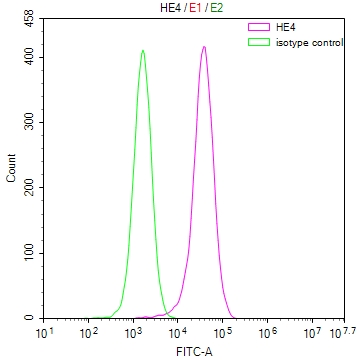
图片:
-
其他:
产品详情
-
产品名称:Rabbit anti-Homo sapiens (Human) CCS Polyclonal antibody
-
Uniprot No.:O14618
-
基因名:CCS
-
宿主:Rabbit
-
反应种属:Human
-
免疫原:Synthesized peptide derived from C-terminal of Human CCS.
-
免疫原种属:Homo sapiens (Human)
-
克隆类型:Polyclonal
-
纯化方式:The antibody was affinity-purified from rabbit antiserum by affinity-chromatography using epitope-specific immunogen.
-
浓度:It differs from different batches. Please contact us to confirm it.
-
产品提供形式:Liquid
-
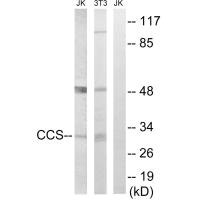
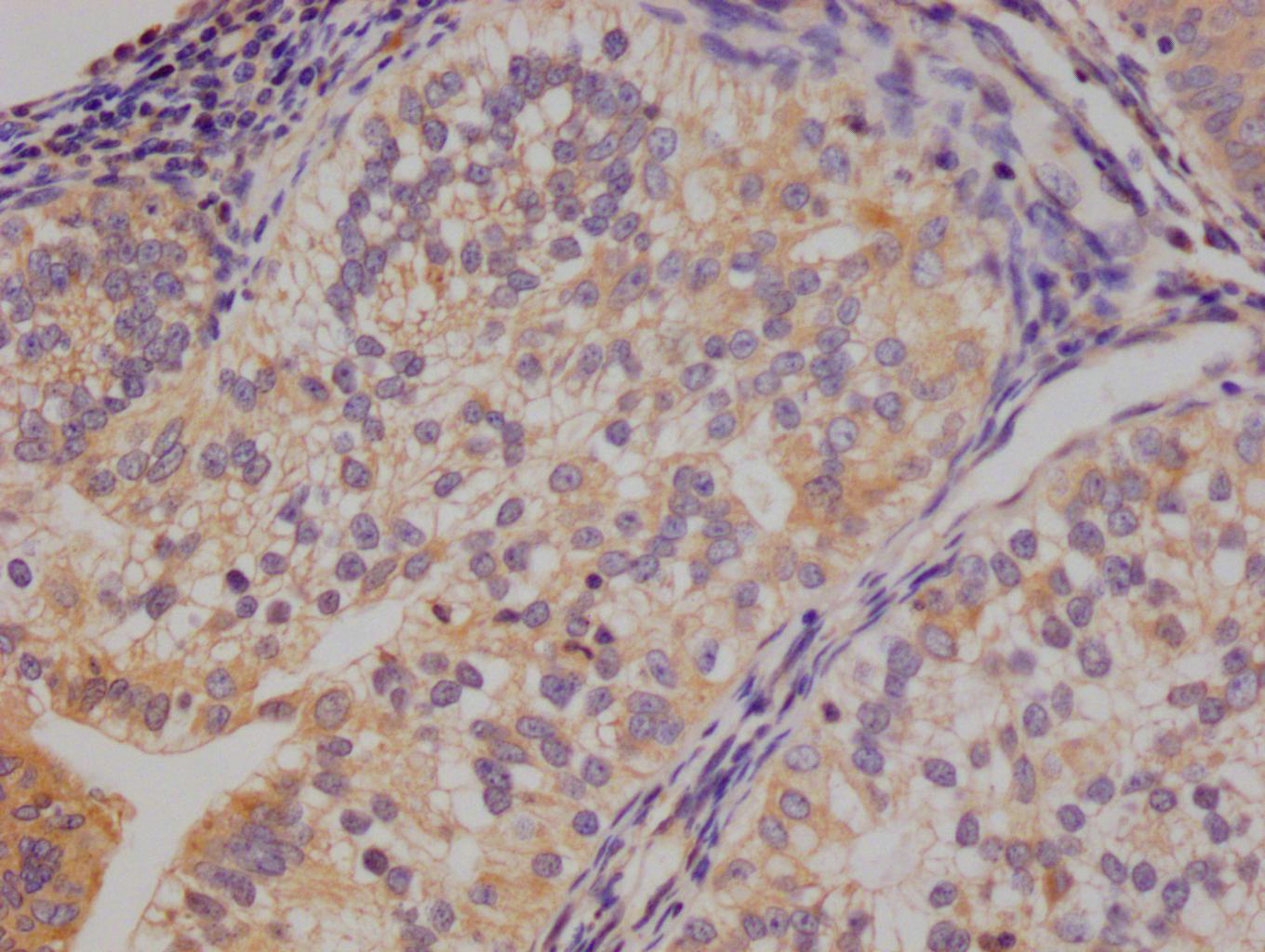
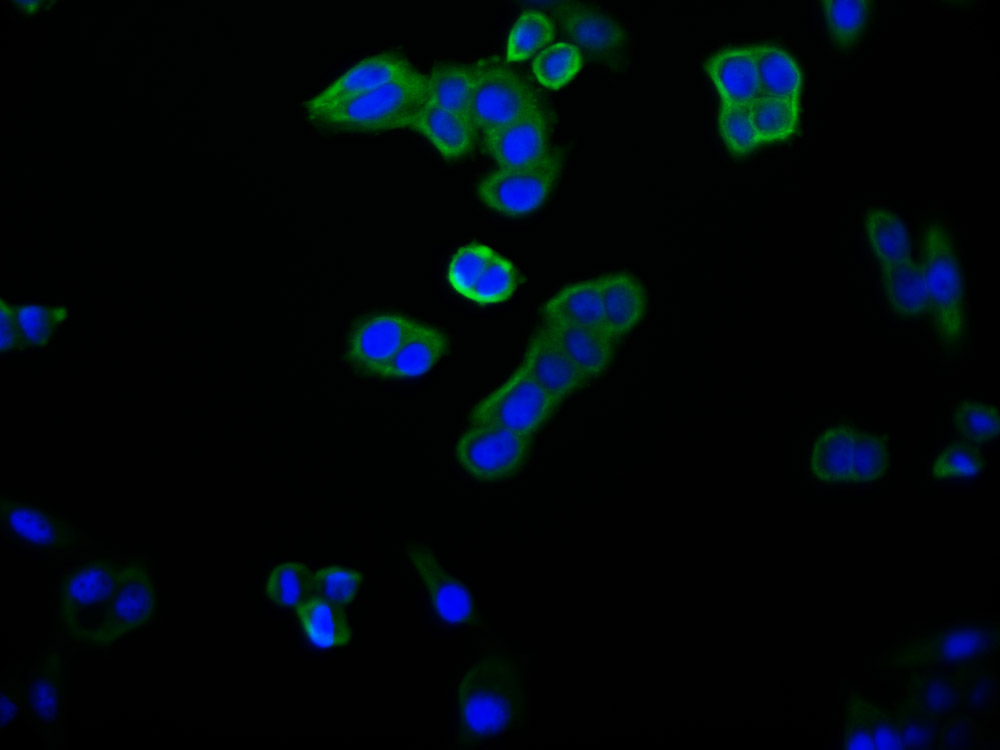
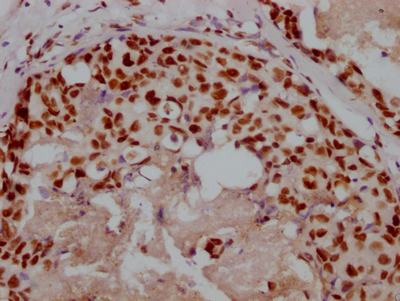
应用范围:ELISA,WB
-
推荐稀释比:
Application Recommended Dilution WB 1:500-1:3000 -
Protocols:
-
储存条件:Upon receipt, store at -20°C or -80°C. Avoid repeated freeze.
-
货期:Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time maybe differs from different purchasing way or location, please kindly consult your local distributors for specific delivery time.
相关产品
靶点详情
-
功能:Delivers copper to copper zinc superoxide dismutase (SOD1).
-
基因功能参考文献:
- In addition to Atox1, the human cytoplasm also contains Cu chaperones for loading of superoxide dismutase 1 (i.e. CCS) and cytochrome c oxidase in mitochondria (i.e. Cox17). [review] PMID: 26745464
- Human cytoplasmic copper chaperones Atox1 and CCS exchange copper ions in vitro PMID: 25673218
- Coexpression of hCCS in the presence of copper restores the correct maturation of the SOD1 mutants and prevents the formation of the unstructured species, confirming that hCCS also acts as a molecular chaperone. PMID: 25429517
- CCS mRNA and protein levels in the serum are not correlated with inflammatory processes. PMID: 24855044
- CTR1 silencing increased the protein levels of copper chaperone ATOX1 and copper chaperone for superoxide dismutase 1 (CCS-1), but decreased copper chaperone for cytochrome c oxidase (COX17). PMID: 24343031
- CCS1 serves as a specialized import receptor in mitochondria that facilitates the import and folding of SOD1 and CCS1. PMID: 24026195
- CCS-dependent copper acquisition and distribution largely occur at membrane interfaces and that this emerging role of the bilayer may reflect a general mechanistic aspect of cellular transition metal ion acquisition. PMID: 24297923
- CCS-1 facilitates copper trafficking to the mitochondria, but does not affect the transfer of copper to the cytochrome c oxidase. PMID: 23900152
- The CCS mutation, p.Arg163Trp, causes reduced SOD1 activity and may impair other mechanisms important for normal Cu homeostasis. PMID: 22508683
- analysis of human superoxide dismutase 1 (hSOD1) maturation through interaction with human copper chaperone for SOD1 (hCCS) PMID: 22869735
- Loss of copper chaperone for superoxide dismutase (CCS) increases amyloid-beta production in both CCS knockout neurons and CCS small-interfering (si)RNA-treated cultured tumor cells. PMID: 20693630
- The results of the present study reveal the plasticity of this multi-domain chaperone in solution and are consistent with an indispensable flexibility necessary for executing its dual functions of metal binding and transfer. PMID: 21722094
- CCS reduces, under non-oxidative conditions, yet facilitates in the presence of H(2)O(2), mitochondrial translocation of inactive SOD1 mutants. PMID: 21354101
- Results describe the identification of the copper chaperone for superoxide dismutase as a mediator of copper delivery to XIAP in cells. PMID: 20154138
- No causative mutations for amyotrophic lateral sclerosis (ALS) gene have been detected in the CCS gene in 20 sporadic ALS patients analyzed, but an intragenic single nucleotide polymorphism has been identified. PMID: 11991808
- Study of site-directed cysteine-to-serine mutants of CCS suggests the formation of a domain III copper cluster within a dimeric or tetrameric protein and further suggest that this cluster may be an important element of CCS copper transfer machinery. PMID: 15736924
- The copper chaperone CCS is responsible for copper insertion into apo-superoxide dismutasse 1. PMID: 16132821
- A mechanism determining the abundance of CCS that is competitive with the process of copper delivery to SOD1 is described, revealing a unique post-translational component of intracellular copper homeostasis. PMID: 16531609
- copper chaperone mRNA levels were reduced in peripheral mononuclear cells after copper supplementation PMID: 17683925
- Measurements of hCCS-induced SOD1 activation were used to show that the C-terminal CXC sequence is both necessary and sufficient for EZn-SOD maturation. PMID: 18393442
- copper chaperone for SOD1 (CCS) facilitates maturation of SOD1 and that CCS overexpression ameliorates intracellular aggregation of mutant SOD1 in vivo. PMID: 18552350
- data suggest that Cys residues in domain 2 of hCCS are involved in the formation, stability, and redox potential of the domain 3 cluster PMID: 19007184
- Incomplete posttranslational modification of nascent superoxide dismutase (SOD)1 polypeptides via trasnsgenic SOD1 copper chaperone (CCS) may be a characteristic shared by familial SOD1 mutants in amyotrophic lateral sclerosis. PMID: 19227972
显示更多
收起更多
-
亚细胞定位:Cytoplasm.
-
蛋白家族:Cu-Zn superoxide dismutase family
-
组织特异性:Ubiquitous.
-
数据库链接:
HGNC: 1613
OMIM: 603864
KEGG: hsa:9973
STRING: 9606.ENSP00000436318
UniGene: Hs.502917
Most popular with customers
-
-
YWHAB Recombinant Monoclonal Antibody
Applications: ELISA, WB, IF, FC
Species Reactivity: Human, Mouse, Rat
-
Phospho-YAP1 (S127) Recombinant Monoclonal Antibody
Applications: ELISA, WB, IHC
Species Reactivity: Human
-
-
-
-
-