CNR1(CB1):内源性大麻素系统ECS经典受体,为糖脂代谢和肿瘤注入全新研究价值!
日期:2023-09-08 11:43:55
最近,丹麦诺和诺德(Novo Nordisk)宣布计划以高达10.75亿美元的价格收购加拿大生物技术公司Inversago Pharma,以获取其基于CB1受体(CB1R)的创新疗法INV-202。这项收购将进一步强化Novo Nordisk在减重领域的战略布局,该公司已拥有了一款强劲的靶向GLP-1R的减重药物Wegovy。INV-202是一种口服CB1R拮抗剂,可以阻断CB1R的活化,从而减少食欲、降低体重、改善血糖和血脂等。
更加令人振奋的是,多款针对CNR1(CB1R/CB1)的药物在肿瘤治疗方面也逐渐显现出潜力。例如,Rimonabant正处于II期临床试验中,用于治疗晚期胃癌,同时AM251也正在进行I期临床试验,以应用于晚期乳腺癌治疗。因此,CB1R作为经典大麻素受体,越来越多的制药公司对这个靶点表现出浓厚兴趣,以探索其在治疗肿瘤和其他代谢疾病方面的潜力!
1. 什么是内源性大麻素系统ECS?
内源性大麻素系统(Endocannabinoid system,ECS)是最重要的能够维持机体内环境稳定的生理系统之一,也是重要的脂质代谢平衡调控因子。ECS主要包括内源性大麻素(AEA、2-AG)及其衍生物,大麻素受体(CNR1、CNR2)及非经典大麻素受体(eg. PPAR、GPR55),各种代谢酶组成(eg. FAAH、MAGL)。ECS成员广泛分布在全身各种组织,如大脑,免疫细胞,结缔组织等。ECS与神经作用、炎症发生、纤维化、疼痛调节等一系列病理生理过程密切相关,参与调控能量代谢,控制胰岛素分泌,维持血脂水平及性激素调控等。近期针对ECS的研究主要围绕它在能量平衡、自体免疫和抗肿瘤中发挥的作用,其中,大麻素受体CNRl的抗肿瘤作用是最近研究的热点 [1-2]。
2. 什么是大麻素Ⅰ型受体基因(CNR1)?
2.1 CNR1的结构
大麻素Ⅰ型受体(Cannabinoid receptor 1,CB1R或CB1、CNR1)作为内源性大麻素信号系统最经典的受体之一,是一种G蛋白耦联的膜受体。CNRl基因位于6q14-q15染色体,包含4个外显子和3个内含子,编码的蛋白CB1R由473个氨基酸组成,N端117位氨基酸形成胞外区,C端401-473位氨基酸构成胞内区,中间有七次跨膜(图1) [3]。胞外区还含有七次跨膜形成的三个亲水性结构域:e1、e2及e3,其中e2被认为是与大麻类物质结合的功能域。大麻素类物质作用于CNR1后,会激活多重细胞内信号转导通路,发挥各种生理和病理功能 [4]。
2.2 CNR1的表达和功能
CNR1被克隆和鉴定的最初,一直被认为其仅在脑中表达。但是大量的后续研究揭示,其不仅在脑中表达,同样在外周的细胞和组织中也表达,比如肺、肝脏、肾、消化道、脂肪组织和骨骼肌 [5-6]。CNR1通过大麻素及其衍生物刺激或结合,激活胞内信号,介导ECS发挥广泛而复杂的生物学作用。例如,CNR1对记忆、认知、运动、情绪等有重要的调节作用;CNR1对腺苷酸环化酶系统中的第二信使AMP水平进行调控,从而影响细胞功能、物质代谢、免疫功能和基因表达等;CNR1还通过细胞内钙调蛋白系统调整钙离子水平,钙离子在细胞功能中具有关键作用,包括影响信号传导、肿瘤发展以及细胞自噬等 [7-8]。近年来,这一系列的发现为CNRl这一发现已逾30年的经典蛋白注入全新的研究价值!
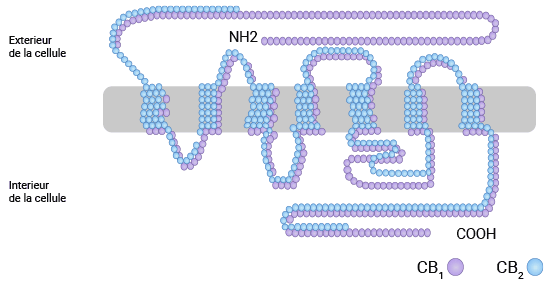
图1. CNR1的结构 [3]
3. CNR1相关的信号通路
3.1 CNR1抑制G蛋白偶联受体信号机制
目前,CNR1(CB1R)作为抑制性G蛋白偶联受体家族成员,其信号转导机制已经得到相对清晰的研究。大麻素受体作用机制主要通过调控G蛋白介导胞内第二信使环磷酸腺苷的产生、钙离子通道、丝裂原活化蛋白激酶MAPKs的活性等相关信号传导通路(图2) [9]。以内源性大麻素(Endocannabinoid,EC)的激活作用为例进行说明。
在脑内,EC作为一种逆向性神经递质,从突触后神经元释放,并作用于突触前膜,从而引发CB1R产生一系列效应。CB1R被激活后,通过Gi/o蛋白信号转导途径,抑制腺苷酸环化酶的活性,降低细胞内cAMP水平。此外,CB1R还通过调节离子通道的活性,促进钾离子(K+)外流,降低细胞内钾离子(Ca2+)水平,并抑制L、N、P/Q型电压依赖性Ca2+通道的活性,降低细胞内Ca2+水平,进而减少突触前膜神经元内γ-氨基丁酸(GABA)、谷氨酸等神经递质的释放 [9]。
该过程还导致CB1R被磷酸化并激活丝裂原激活蛋白激酶,磷酸化的CB1R与β-arrestin分子紧密联系,参与调节G蛋白偶联受体信号。此外,在特定环境中,CB1R还能通过Gs蛋白信号途径激活腺苷酸环化酶,增加细胞内cAMP含量。再者,CB1R还能通过Gq蛋白信号途径激活Gq偶联的胞内钙离子通道,这种效应具有细胞选择性。CB1R介导的药理效应在不同组织中存在差异,可能是因为CB1R能够与其他受体形成寡聚体/二聚体结构 [9-10]。
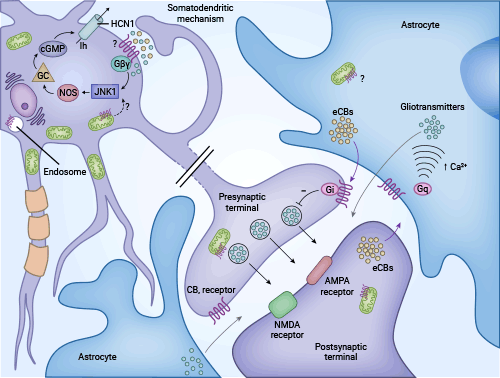
图2. CNR1抑制G蛋白偶联受体信号机制 [9]
3.2 CNR1协同大麻素系统的抗肿瘤机制
大麻素系统在抗癌作用上机制十分复杂。一般认为,大麻素通过与细胞膜上的受体CNRl(CB1)或CNR2(CB2)结合,导致细胞内神经酰胺合成增加,从而激活ERK通路、p38MAPK、PI3K/PKB信号通路,促进细胞凋亡 [11]。此外,大麻素与受体结合后还能通过ERK和PI3K/PKB信号通路激活P27/KIP1、Cyclins、Cdks信号通路,从而抑制细胞增值 [12]。另外,大麻素与受体CNRl或CNR2结合抑制AKT/PKB磷酸化以及MMP-2、MMP-9的分泌,从而抑制肿瘤细胞的迁移 [13-14]。
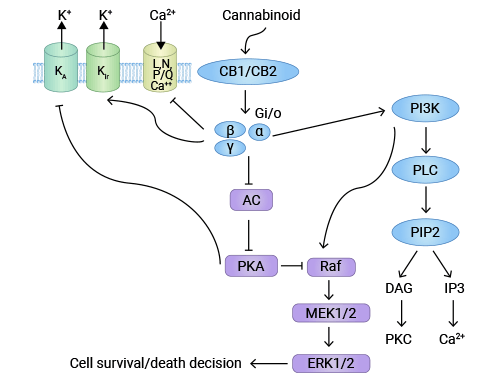
图3. CNR1协同大麻素系统的抗肿瘤机制 [11]
4. CNRl在疾病中的作用
4.1 CNR1与肿瘤
CNR1(CB1R)在不同肿瘤中的作用广泛而复杂。CNR1在多种肿瘤中表达上调,包括前列腺癌 [15]、胰腺癌 [16]、霍奇金淋巴瘤 [17]、肾透明细胞癌 [18]和肾嗜酸细胞癌 [19]等,然而,在低分化肝细胞癌及结肠癌中表达下调 [20-21]。在胰腺癌中,低表达CNR1的患者疼痛较轻,预后较好 [16];在前列腺癌中,高表达CNR1与前列腺肿瘤恶性程度高及预后差相关 [15];但是,在肝癌中,高表达CNR1患者预后较好 [20]。因此,CNR1在不同肿瘤发生发展中的作用仍然需要深入探索。
CNR1还与肿瘤的生长、血管发生和迁移有关,可发挥抗癌作用。目前所有围绕内源性大麻素系统抑癌作用的研究都是利用大麻素类似物—大麻素激动剂来完成的。在人类乳腺癌肺转移鼠模型中应用大麻素受体激动剂(JWH133和Win55, 212-2)可抑制原发肿瘤的生长,降低肺转移病灶的大小和数量 [22-23]。在雄激素抵抗的PC-3前列腺癌细胞鼠模型中,在肿瘤周围直接注射JWH015能明显缩小肿瘤 [24]。大麻素可通过损害机体抗肿瘤免疫能力,促进IL-4和IL-10等分泌,进而促进乳腺癌的发生发展 [25]。在黑色素瘤和肾癌研究中,CNR1特异性抑制剂AM251可明显抑制肿瘤细胞的增殖,促进肿瘤细胞的凋亡与G2/M周期阻滞 [26-27]。
4.2 CNR1与糖脂代谢相关疾病
CNR1(CB1R)过表达会影响许多疾病,例如肥胖、代谢综合征、糖尿病、脂肪肝等 [28-32]。利莫那班(Rimonabant)是CNR1选择性受体拮抗剂,通过减低中枢神经系统受体的活性,从而降低患者的食欲、进食量、减少肝内脂肪生成等。同时,Rimonabant可通过抑制外周组织(如骨骼肌、脂肪组织等)受体的过度表达,发挥着减肥作用,并且改善胰岛素抵抗和脂肪组织的代谢。该抑制剂在临床上已被广泛用于减肥治疗,可有效改善与肥胖症相关疾病的危险因素 [33-34]。
关于抑制CB1R后产生上述效应的具体机制仍不清楚。有研究指出,CB1受体抑制剂可以改善胰岛素抵抗,其机制可能是激活受体CB1R可使肝脏脂肪合成转录因子固醇调节元件结合蛋白-lc、乙酰辅酶A羧化酶-1和脂肪酸合成酶基因表达增高,引起小鼠肥胖 [35]。过高的脂肪酸水平会使胰岛素刺激的葡萄糖摄取功能受损,导致血糖升高,慢性高血糖引起胰岛B细胞功能的减退。总之,激动CB1R可以增加体重,升高血脂,加重胰岛素抵抗和使胰岛B细胞功能减退,抑制CB1R可逆转此效应,这为糖脂代谢相关疾病的治疗提供了新的思路。
4.3 CNR1与其它疾病
此外,CNR1在情感障碍、抑郁症、肠道疾病、银屑病和疼痛方面的作用也受到了一些关注 [36-40]。一些研究发现,CNR1基因的变异或是抑郁症发生的风险因素,尤其是在女性和年轻人中 [41];CNR1还在调节肠道的运动、分泌、感觉和免疫反应方面发挥着重要的作用,这些方面都与肠易激综合征和溃疡性结肠炎的发病机制有关 [37];银屑病患者血清和皮损组织中CNR1和CNR2表达升高,且与患者病情严重程度有关 [38];此外,CNR1激动剂对神经性疼痛、癌症相关疼痛和其他慢性非癌性疼痛有一定的镇痛效果,但也有一些不良反应,如精神障碍、恶心和嗜睡等 [39-40]。
5. CNR1的临床药物研究前景
目前,全球已经上市了两种基于CNR1的靶向药物,用于治疗肥胖、癌症疼痛等多种疾病。特别值得一提的是,大麻二酚/四氢大麻酚(Cannabidiol/Dronabinol)药物已获得欧盟认定为孤儿药资格。目前,CNR1已有100多个临床在研项目,这些CNR1相关药物主要包括小分子化合物,单克隆抗体和诊断用放射药物等。CNR1作为参与多种生理功能的大麻素受体,如情绪、食欲、脂质代谢、认知和疼痛等,其临床研究领域涵盖内分泌、神经、皮肤、代谢、肿瘤等多个领域。在这些领域,研究人员主要利用CNR1的拮抗剂、反向激动剂、激动剂或调节剂来治疗各种疾病,如肥胖、糖尿病、疼痛、焦虑、恶病质、药物中毒、肿瘤等。随着对CNR1的深入了解和创新药物的不断开发,CNR1已成为治疗肿瘤和其他代谢疾病的重要靶点之一!
为鼎力协助科研和药企人员针对CNR1在多种糖脂代谢相关疾病和肿瘤中的临床应用研究,CUSABIO推出CNR1活性蛋白(Code: CSB-MP005678HU),助力您在CNR1机制方面的研究或其潜在临床价值的探索。
Cannabinoid receptor 1(CNR1)蛋白
● Recombinant Human Cannabinoid receptor 1(CNR1)-VLPs (Active)
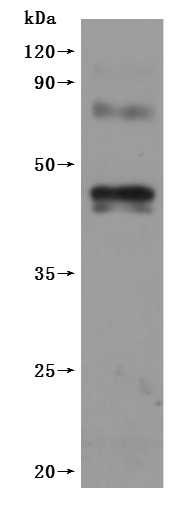
The high specifity is detected by Mouse anti-6*His monoclonal antibody.
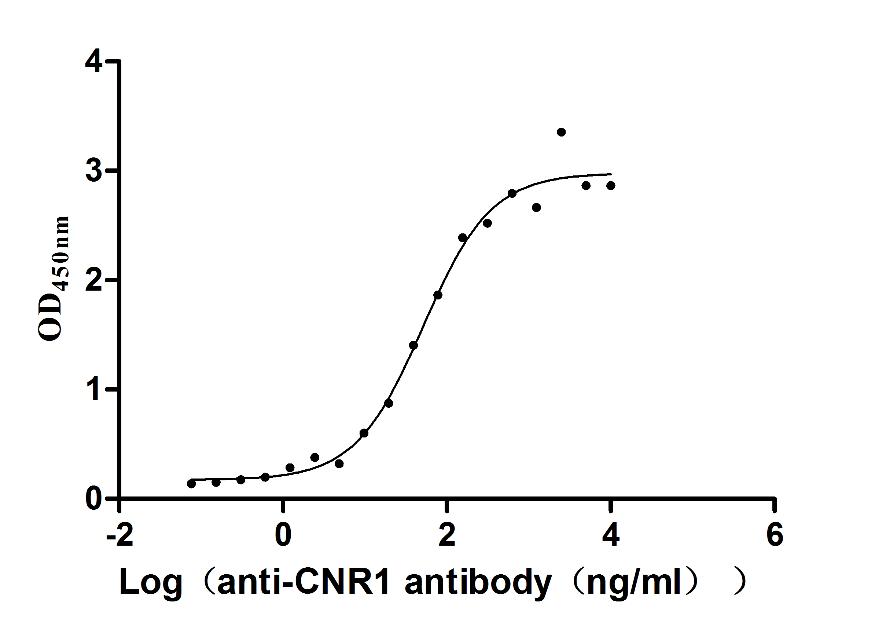
Immobilized Human CNR1 at 10 μg/ml can bind Anti-CNR1 recombinant antibody (CSB-RA005678MA01HU), the EC50 is 41.72-63.54 ng/mL.
Cannabinoid receptor 1(CNR1)抗体
CNR1 Recombinant Monoclonal Antibody (Code: CSB-RA005678MA01HU)
参考文献:
[1] Battista, Natalia, et al. "The endocannabinoid system: an overview." Frontiers in behavioral neuroscience (2012): 9.
[2] Lu, Hui-Chen, and Ken Mackie. "Review of the endocannabinoid system." Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 6.6 (2021): 607 -615.
[3] Jourdan, Tony. Impact du système endocannabinoïdien sur la physiologie de l'obésité: effets de l'antagonisme des récepteurs CB1 sur le métabolisme glucido-lipidique de la souris obèse. Diss. Dijon, 2010.
[4] Benyamina, Amine, et al. "CNR1 gene polymorphisms in addictive disorders: a systematic review and a meta-analysis." Addiction biology 16.1 (2011): 1-6.
[5] Ujike, H., et al. "CNR1, central cannabinoid receptor gene, associated with susceptibility to hebephrenic schizophrenia." Molecular psychiatry 7.5 ( 2002): 515-518.
[6] Tao, Ran, et al. "Cannabinoid receptor CNR1 expression and DNA methylation in human prefrontal cortex, hippocampus and caudate in brain development and schizophrenia." Translational psychiatry 10.1 (2020): 158.
[7] Szafran, Brittany N., et al. "Cnr1-/- has minimal impact on chlorpyrifos-mediated effects in the mouse endocannabinoid system, but it does alter lipopolysaccharide-induced cytokine levels in splenocytes." Chemico-Biological Interactions 375 (2023): 110425.
[8] Huynh, Karina. "CNR1 antagonism attenuates cannabis-induced atherosclerosis." Nature Reviews Cardiology 19.7 (2022): 432-432.
[9] Busquets-Garcia, Arnau, Jaideep Bains, and Giovanni Marsicano. "CB1 receptor signaling in the brain: extracting specificity from ubiquity." Neuropsychopharmacology 43.1 (2018): 4-20.
[10] Lutz, Beat. "Neurobiology of cannabinoid receptor signaling." Dialogues in clinical neuroscience (2022).
[11] Chakravarti, Bandana, Janani Ravi, and Ramesh K. Ganju. "Cannabinoids as therapeutic agents in cancer: current status and future implications." Oncotarget 5.15 (2014): 5852.
[12] Galve-Roperh, Ismael, et al. "Mechanism of extracellular signal-regulated kinase activation by the CB1 cannabinoid receptor." Molecular pharmacology 62.6 (2002): 1385-1392.
[13] Hong, Jun, et al. "CB1 cannabinoid receptor agonist inhibits matrix metalloproteinase activity in spinal cord injury: a possible mechanism of improved recovery." Neuroscience letters 597 (2015): 19-24.
[14] Adhikary, Sabina, et al. "Signaling through cannabinoid receptor 2 suppresses murine dendritic cell migration by inhibiting matrix metalloproteinase 9 expression." Blood, The Journal of the American Society of Hematology 120.18 (2012): 3741-3749.
[15] Chung, Sui Chu, et al. "A high cannabinoid CB1 receptor immunoreactivity is associated with disease severity and outcome in prostate cancer." European Journal of Cancer 45.1 (2009): 174-182.
[16] Michalski, Christoph W., et al. "Cannabinoids in pancreatic cancer: correlation with survival and pain." international journal of cancer 122.4 (2008). : 742-750.
[17] Benz, Alexander H., et al. "Expression and functional relevance of cannabinoid receptor 1 in Hodgkin lymphoma." PloS one 8.12 (2013): e81675.
[18] Larrinaga, Gorka, et al. "Cannabinoid CB1 receptor is downregulated in clear cell renal cell carcinoma." Journal of Histochemistry & Cytochemistry 58.12 (2010): 1129-1134.
[19] Varricchi, Gilda, et al. "Innate effector cells in angiogenesis and lymphangiogenesis." current opinion in immunology 53 (2018): 152-160.
[20] Larrinaga, Gorka, et al. "Cannabinoid CB1 receptor is expressed in chromophobe renal cell carcinoma and renal oncocytoma." Clinical Biochemistry 46.7 -8 (2013): 638-641.
[21] Argaw, Anteneh, et al. "Concerted action of CB1 cannabinoid receptor and deleted in colorectal cancer in axon guidance." Journal of Neuroscience 31.4 ( 2011): 1489-1499.
[22] Qamri, Zahida, et al. "Synthetic cannabinoid receptor agonists inhibit tumor growth and metastasis of breast cancer." Molecular cancer therapeutics 8.11 (2009): 3117-3129.
[23] Sophocleous, Antonia, et al. "Bone cell-autonomous contribution of type 2 cannabinoid receptor to breast cancer-induced osteolysis." Journal of Biological Chemistry 290.36 (2015): 22049-22060.
[24] Olea-Herrero, N., et al. "Inhibition of human tumour prostate PC-3 cell growth by cannabinoids R (+)-Methanandamide and JWH-015: involvement of CB2." British journal of cancer 101.6 (2009): 940-950.
[25] McKallip, Robert J., Mitzi Nagarkatti, and Prakash S. Nagarkatti. "Δ-9-tetrahydrocannabinol enhances breast cancer growth and metastasis by suppression of the antitumor immune response." The Journal of Immunology 174.6 (2005): 3281-3289.
[26] Kenessey, István, et al. "Revisiting CB1 receptor as drug target in human melanoma." Pathology & Oncology Research 18 (2012): 857-866.
[27] Larrinaga, Gorka, et al. "Cannabinoid CB1 receptor is downregulated in clear cell renal cell carcinoma." Journal of Histochemistry & Cytochemistry 58.12 (2010): 1129-1134.
[28] Tam, Joseph, et al. "Endocannabinoids in liver disease." Hepatology 53.1 (2011): 346-355.
[29] Pacher, Pál, and George Kunos. "Modulating the endocannabinoid system in human health and disease-successes and failures. "The FEBS journal 280.9 (2013): 1918-1943.
[30] Kunos, George, and Joseph Tam. "The case for peripheral CB1 receptor blockade in the treatment of visceral obesity and its cardiometabolic complications." British journal of pharmacology 163.7 (2011): 1423-1431.
[31] Cota, Daniela. "CB1 receptors: emerging evidence for central and peripheral mechanisms that regulate energy balance, metabolism, and cardiovascular health." Diabetes/metabolism research and reviews 23 7 (2007): 507-517. health." Diabetes/metabolism research and reviews 23.7 (2007): 507-517.
[32] Leal, Ermelindo C., et al. "Diabetes and Cannabinoid CB1 receptor deficiency promote similar early onset aging-like changes in the skin." Experimental gerontology 154 (2021): 111528.
[33] Henness, Sheridan, Dean M. Robinson, and Katherine A. Lyseng-Williamson. "Rimonabant." Drugs 66 (2006): 2109-2119.
[34] Christensen, Robin, et al. "Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomized trials." The Lancet 370.9600 (2007). 1706-1713.
[35] Schwabe, Robert F. "Endocannabinoids promote hepatic lipogenesis and steatosis through CB1 receptors." (2005): 959-961.
[36] Witkin, Jeffrey M., et al. "A therapeutic role for cannabinoid CB1 receptor antagonists in major depressive disorders." Trends in pharmacological sciences 26.12 (2005): 609-617.
[37] Galiazzo, Giorgia, et al. "Localization of cannabinoid receptors CB1, CB2, GPR55, and PPARα in the canine gastrointestinal tract." Histochemistry and cell biology 150 (2018): 187-205.
[38] Wilkinson, Jonathan D., and Elizabeth M. Williamson. "Cannabinoids inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism and have a potential therapeutic value in the treatment of psoriasis." Journal of dermatological science 45.2 (2007): 87-92.
[39] Clayton, N., et al. "CB1 and CB2 cannabinoid receptors are implicated in inflammatory pain." Pain 96.3 (2002): 253-260.
[40] Davis, Mellar P. "Cannabinoids in pain management: CB1, CB2 and non-classic receptor ligands." expert opinion on investigational drugs 23.8 (2014). 1123-1140.
[41] Ostlund, Isaac, et al. "Chronic Δ9-tetrahydrocannabinol impact on plasticity, and differential activation requirement for CB1-dependent long-term depression in ventral tegmental area GABA neurons in adult versus young mice." Frontiers in Neuroscience 16 (2023): 1067493.












