CDCP1蛋白:一种致癌信号通路的关键枢纽,抗肿瘤药物新兴靶点!
日期:2023-08-25 09:22:05
最近,哈佛医学院的研究者在Nature Communications发表了一篇题为“The blood proteome of imminent lung cancer diagnosis”的论文。采用高通量蛋白质组学方法,研究人员从吸烟者的血液样本中筛选出与即将诊断为肺癌的风险相关的蛋白质,其中包括CEACAM5、MUC-16、IL6、CXCL9、CXCL13和CDCP1 [1]。CDCP1是一种新型的肿瘤治疗靶点,目前有多种针对它的药物在开发中,主要针对实体瘤中高表达CDCP1的肿瘤细胞。这些药物能够抑制肿瘤细胞的迁移和侵袭,同时保护正常细胞,特别是造血干细胞。因此,CDCP1作为一种重要的致癌信号通路的调节因子,具有巨大的药物开发潜力,有望为肿瘤患者带来新的治疗方案。
1. 什么是CDCP1?
CUB结构域包含蛋白1(CUB-domain containing protein 1,CDCP1)是I类单次跨膜糖蛋白,也被称CD318、SIMA135、gp140或TRASK。Scherlmostageerm等研究者首次于2001年使用代表性差异分析和cDNA芯片技术鉴定,报告了一种新的人类肿瘤相关基因CDCP1。全长CDCP1蛋白含有836个氨基酸,包含29个氨基酸的氨基端信号肽、细胞外结构域、跨膜结构域和胞质结构域。CDCP1胞外区有三个未知功能的CUB样结构域,其最有可能参与细胞黏附或与细胞外基质相互作用。CDCP1可以被蛋白酶(如ADAM9)水解,形成同源二聚,进而与Src和PKCδ等蛋白发生酪氨酸磷酸化反应,从而启动转移活性(图1)[2-4]。
CDCP1在体内多种组织中的干细胞或前体细胞检测到表达,如造血细胞干细胞祖细胞、神经祖细胞等。最近的研究发现,CDCP1的表达失调与多种肿瘤的发生相关,并且驱动或参与多种恶性肿瘤的迁移及侵袭过程,在肿瘤发生发展中扮演极其重要的角色。CDCP1可通过调节肿瘤内细胞因子(如IL-2、HLA-B19)活性影响肿瘤免疫反应,进而影响治疗效果。CDCP1正成为目前抗肿瘤药物研发领域的热门靶点之一 [5-6]。
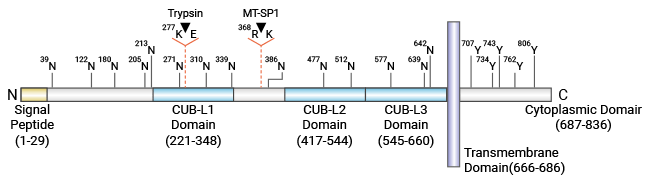
图1. CDCP1结构 [2]
2. CDCP1在肿瘤中的相关作用机制
目前,SFKs、PKCδ与CDCP1的酪氨酸磷酸化相互作用机制正受到关注。相关研究显示,CDCP1蛋白通过调控下游络氨酸磷酸化信号通路,如PI3K/Akt、PKC5、SRC和ERK/MAPK等,在促进肿瘤侵袭和转移中发挥重要作用 (图2) [7]。
在前列腺癌细胞中,CDCP1基因的表达通常被雄激素抑制,保持相对较低的水平。然而,在雄激素非依赖型去势抵抗性前列腺癌(Castration-resistant prostate cancer,CRPC)中,雄激素剥夺过程导致CDCP1蛋白水平上调,同时激活了对应的Akt和SRC/MAPK信号通路,表明CDCP1蛋白的表达水平变化可能与CRPC的进展相关 [8]。此外,CDCP1缺失可调控CDK5,这种新机制动态调节非贴壁细胞中的整合素β1(ITGB1),促进晚期前列腺癌患者的血管扩散 [9]。因此,了解基因转录激活机制以及CDCP1蛋白介导的细胞信号通路在疾病中的作用,有助于指导抑制CDCP1和开发前列腺癌创新疗法的研究。
在结肠癌中,CDCP1鉴定为Wnt信号通路的正调控因子。CDCP1促进Wnt信号转导的关键调节子,β-catenin和E-cadherin转运到细胞核 [10]。在三阴性乳腺癌中,PDGF-BB通过激活PDGFRβ,促进了ERK1/2信号通路的激活,并增加了CDCP1蛋白的表达 [11]。在肺癌中,ADAM9通过激活EGFR信号通路下调miR-1,从而增强CDCP1的表达,促进肺癌的进展 [12]。在肾癌中,HIF-2α的过表达会促进肿瘤异种移植物的生长,并增强CDCP1的表达和酪氨酸磷酸化,表明了CDCP1是转移性癌症的生物标志物和潜在治疗靶标 [12]。
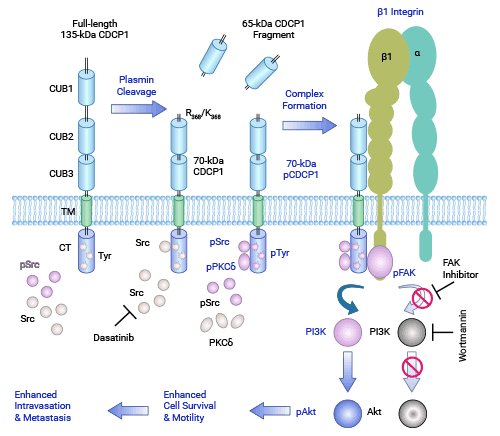
图2. CDCP1在肿瘤中的相关作用机制 [7]
3. CDCP1在肿瘤中的作用
3.1 CDCP1和前列腺癌
前列腺癌(Prostate Cancer,PCa)是男性常见肿瘤,雄激素剥夺疗法(Androgen Deprivation Therapy,ADT)抑制雄激素的产生或阻断雄激素受体(Androgen Receptor,AR)信号,在治疗晚期前列腺癌和预防前列腺癌复发方面具有疗效。然而,大多数患者会出现药物抵抗,最终发展为雄激素非依赖型去势抵抗性前列腺癌(CRPC)。因此,CRPC仍是一种具有临床挑战性的晚期癌症 [14]。
据报道,CDCP1在AR-阴性CRPC细胞中高表达,且与BRD4和CBP/p300表达呈正相关,作为PCa细胞信号传导通路上游的调控因子;靶向BRD4和CBP/p300可以下调EGFR、c-Myc、SRC、ERK/p-ERK、Akt/p-Akt等致癌信号通路标志物的水平;BRD4和CBP/p300结合到CDCP1基因的启动子和增强子区域,调控H3K27ac的水平和CDCP1基因的转录激活;双重抑制剂NEO2734可以有效地抑制CDCP1基因的表达,抑制AR-阳性和AR-阴性CRPC细胞生长 (图3) [15] 。此外,CDCP1与抑癌基因PTEN的缺失,协同促进了转移性前列腺癌的发生 [16]。
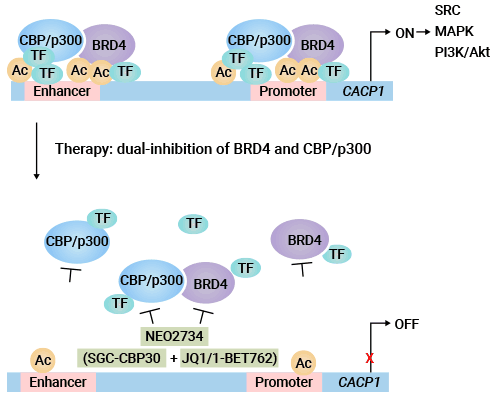
图3. 双重抑制剂以靶向CDCP1蛋白所介导的雄激素非依赖型去势抵抗性前列腺癌CRPC [15]
3.2 CDCP1和结肠癌
CDCP1是一种与结直肠癌的预后相关的分子标志物,其高表达的患者比低表达的患者具有更短的总生存期和无病生存期。在体外实验中,通过基因编辑技术沉默CDCP1的表达能够有效抑制HCT116细胞的迁移和侵袭能力,说明CDCP1的过度表达促进了癌细胞的转移和侵袭。此外,通过整合基因组学分析发现RHO关联卷曲螺旋结合蛋白激酶1(ROCK1)是CDCP1调控迁移和侵袭的重要下游信号分子 [17]。
3.3 CDCP1和肺癌
肺癌是常见的恶性肿瘤之一,其中非小细胞肺癌(NSCLC)占肺癌的70%-80%以上。对于接受EGFR TKI治疗的EGFR突变阳性NSCLC患者中,研究者发现,CDCP1是影响患者无进展生存期(Progression-Free Survival,PFS)和总生存期(Overall Survival,OS)的独立负面预后因素 [18-19]。相关研究发现,临床标本中肺癌患者ADAM9和CDCP1的高水平与高死亡相关。此外,ADAM9可激活EGFR信号通路,增强CDCP1的表达,促进肺癌 [12, 20]。
3.4 CDCP1和胰腺癌
在胰腺癌中,CDCP1的表达水平很高。研究表明,AHCC是一种从蕈类植物中提取的活性成分,它可以通过下调CDCP1的表达,抑制胰腺癌细胞的恶性进展。另一方面,CDCP1可以通过其细胞外的CUB2结构域与另一个CDCP1分子结合,形成同型复合物。这种复合物可以激活SFK信号分子,从而促进癌细胞的迁移。因此,研究人员利用麦芽糖结合蛋白标记的重组CUB2域蛋白(rMBPCUB2),干扰CDCP1之间的结合,有效地减少了同型复合物的数量。这说明,阻断CDCP1的同型结合位点是一种潜在的治疗CDCP1相关肿瘤的策略 [21]。
3.5 CDCP1和乳腺癌
在乳腺癌中,实验研究确定了CDCP1是HER2信号的调节剂。当HER2和CDCP1同时过度表达时,会增加乳腺癌细胞的转化、迁移和肿瘤形成能力 [22]。在三阴性乳腺癌(TNBC)中,有研究揭示CDCP1的蛋白水平受到FBXL14的控制。FBXL14是一种泛素连接酶,它可以促进CDCP1的泛素化,使其被蛋白酶体降解。这样,FBXL14就抑制了CDCP1的稳定性,从而影响了CDCP1参与的乳腺癌转移相关的基因表达 [23]。这项研究首次揭示了影响CDCP1稳定性的分子机制,并发现这种机制比基因表达水平更能准确地预测乳腺癌患者的预后。
3.6 CDCP1和其它癌症
CDCP1还在其他多种癌症中有高表达,如喉癌 [24]、宫颈癌 [25]、肾癌 [13]、肝细胞癌 [26]、慢性髓系白血病(CML)[27]和急性髓系白血病(AML) [28-29]等。以喉癌为例,一项研究检测了喉癌组织和癌旁组织中的MMP-9、CDCP1和NLK的表达。结果发现,喉癌组织中这三种蛋白的表达都高于癌旁组织,尤其是NLK。NLK是一种激酶,它可以调节细胞的信号传导 [24]。
研究人员用siRNA干扰了喉癌细胞Hep-2中的NLK表达,发现这样可以降低Hep-2细胞的增殖和侵袭能力,增加Caspase-3的活性。Caspase-3是一种凋亡相关的酶。同时,干扰NLK表达也可以降低MMP-9和CDCP1的表达。这些结果说明,NLK可能通过影响MMP-9和CDCP1的表达,参与喉癌细胞的增殖、凋亡和侵袭过程 [24]。
4. CDCP1的临床在研药物
目前,已有多家国外公司投入CDCP1药物的研发。这些公司包括Chiome Bioscience, Inc.,UCSF Innovation Ventures,Miltenyi Biotec, Inc.,和Heidelberg Pharma Research GmbH等。他们研发的药物形式有单抗、抗体药物缀合物(ADC)和CAR T等。其中,Chiome Bioscience公司开发了一种新型的抗CDCP1抗体药物缀合物(h14A043-ATAC),它已经进入了临床前阶段,并且对多种实体癌显示出了很好的效果。该公司还开发了一种CDCP1单抗抑制剂(PCDC),也在进行临床前试验。目前,国内使用的CDCP1抗体制剂大多是从国外进口的。因此,我们有必要自主研制CDCP1抗体制剂,以满足国内在肿瘤发生发展、免疫诊断和靶向治疗方面对CDCP1的需求。
为鼎力协助科研和药企人员针对CDCP1在肿瘤等疾病中的临床应用研究,CUSABIO推出CDCP1多个种属活性蛋白(CSB-MP884474HU;CSB-MP719456MO;CSB-MP4569MOV),助力您在CDCP1机制方面的研究或其潜在临床价值的探索。
CDCP1蛋白
● Recombinant Human CUB domain-containing protein 1(CDCP1),partial (Active) (Code: CSB-MP884474HU)
Purity was greater than 95% as determined by SDS-PAGE.
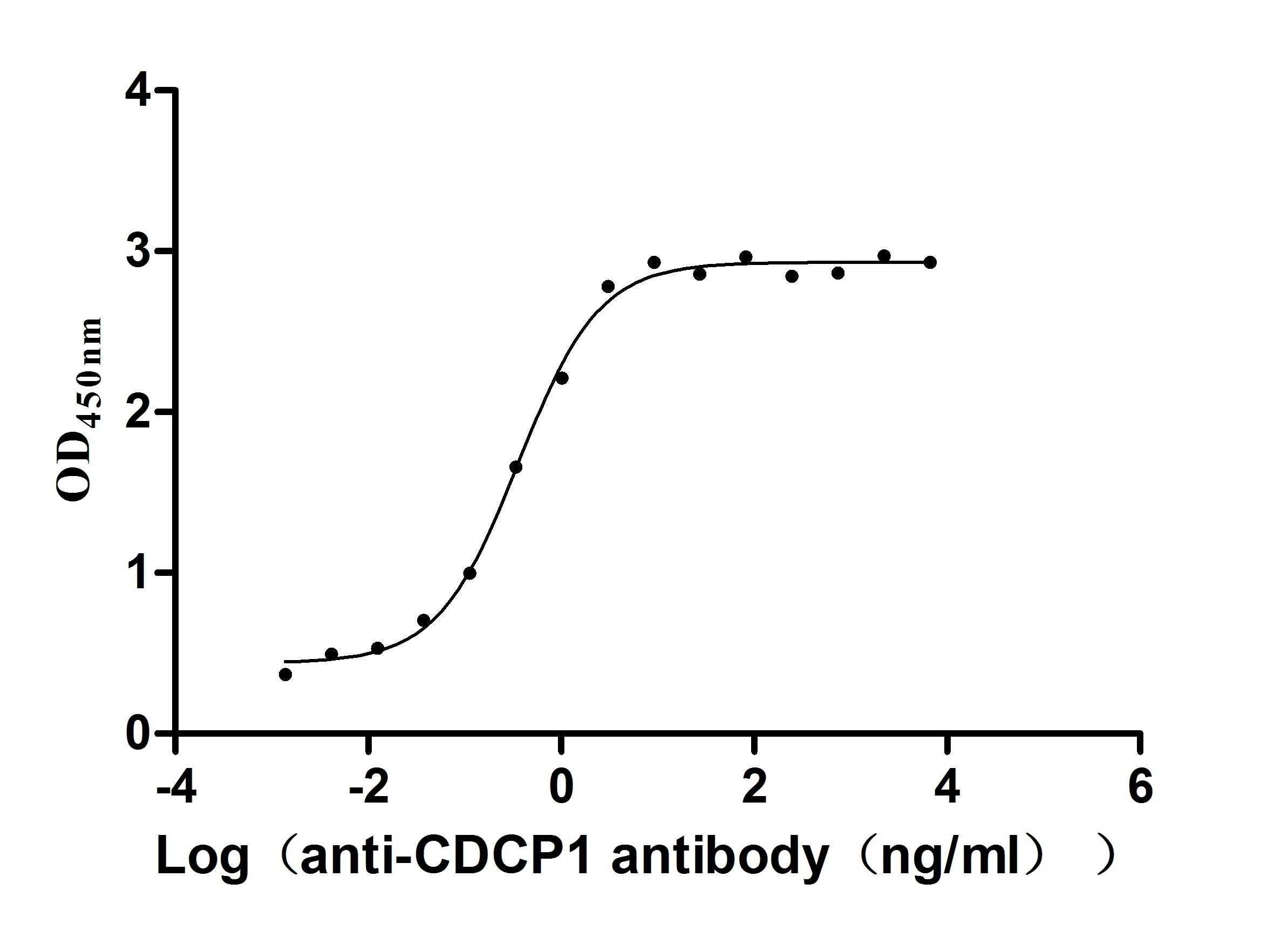
Immobilized Human CDCP1 at 2μg/mL can bind Anti-CDCP1 recombinant antibody (CSB-RA884474MA1HU), the EC50 is 0.2943-0.4429 ng/mL.
● Recombinant Mouse CUB domain-containing protein 1(Cdcp1),partial (Active) (Code: CSB-MP719456MO)
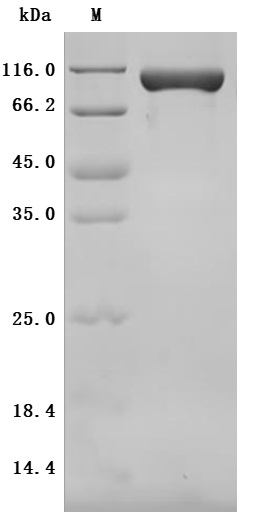
Purity was greater than 95% as determined by SDS-PAGE.
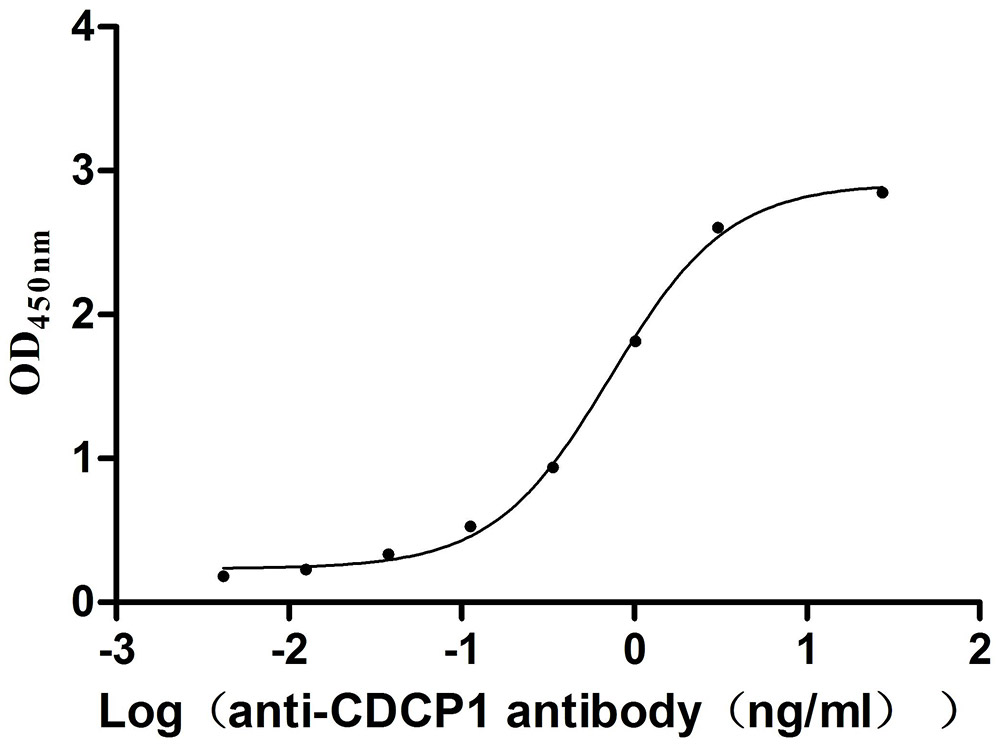
Immobilized Mouse Cdcp1 at 2 μg/mL can bind Anti-CDCP1 recombinant antibody (CSB-RA884474MA1HU), the EC50 is 0.6397-0.8369 ng/mL.
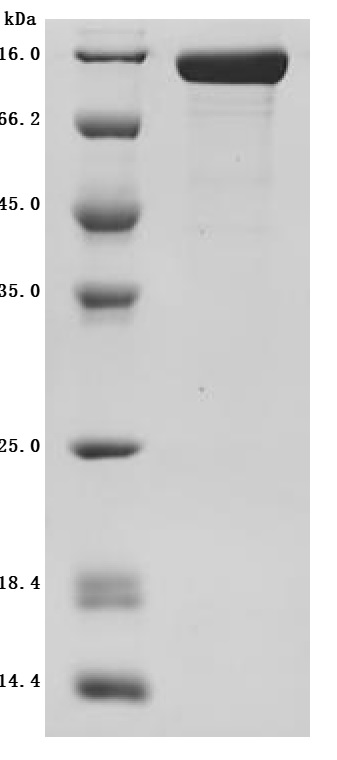
Purity was greater than 95% as determined by SDS-PAGE.

Immobilized Macaca fascicularis CDCP1 at 2μg/mL can bind Anti-CDCP1 recombinant antibody (CSB-RA884474MA1HU),the EC50 is 1.861-2.330 ng/mL.
参考文献:
[1] Albanes, Demetrius, et al. "The blood proteome of imminent lung cancer diagnosis." Nature Communications 14.1 (2023).
[2] Wortmann, Andreas, et al. "The cell surface glycoprotein CDCP1 in cancer-insights, opportunities, and challenges." IUBMB life 61.7 ( 2009): 723-730.
[3] Murakami, Yuichi, et al. "AXL/CDCP1/SRC axis confers acquired resistance to osimertinib in lung cancer." Scientific Reports 12.1 (2022): 8983.
[4] Uekita, Takamasa, and Ryuichi Sakai. "Roles of CUB domain-containing protein 1 signaling in cancer invasion and metastasis." Cancer science 102.11 (2011): 1943-1948.
[5] Qi, Xiao, et al. "CDCP1: A promising diagnostic biomarker and therapeutic target for human cancer." Life Sciences 301 (2022): 120600.
[6] Hooper, John D., et al. "Subtractive immunization using highly metastatic human tumor cells identifies SIMA135/CDCP1, a 135 kDa cell surface phosphorylated glycoprotein antigen." Oncogene 22.12 (2003): 1783-1794.
[7] Casar, B., et al. "In vivo cleaved CDCP1 promotes early tumor dissemination via complexing with activated β1 integrin and induction of FAK/PI3K/Akt motility signaling." Oncogene 33.2 (2014): 255-268.
[8] Alajati, Abdullah, et al. "CDCP1 overexpression drives prostate cancer progression and can be targeted in vivo." the Journal of clinical investigation 130.5 (2020): 2435-2450.
[9] Pollan, Sara G., et al. "Regulation of inside-out β1-integrin activation by CDCP1." Oncogene 37.21 (2018): 2817-2836.
[10] He, Yaowu, et al. "CDCP1 enhances Wnt signaling in colorectal cancer promoting nuclear localization of β-catenin and E-cadherin." Oncogene 39.1 (2020) : 219-233.
[11] Forte, Luca, et al. "The PDGFRβ/ERK1/2 pathway regulates CDCP1 expression in triple-negative breast cancer." BMC cancer 18 (2018): 1-11.
[12] Chiu, Kuo-Liang, et al. "ADAM9 enhances CDCP1 by inhibiting miR-1 through EGFR signaling activation in lung cancer metastasis." Oncotarget 8.29 (2017). : 47365.
[13] Emerling, Brooke M., et al. "Identification of CDCP1 as a hypoxia-inducible factor 2α (HIF-2α) target gene that is associated with survival in clear cell renal cell carcinoma patients." Proceedings of the National Academy of Sciences 110.9 (2013): 3483-3488.
[14] Chandrasekaran, Balaji, et al. "Antiandrogen-Equipped Histone Deacetylase Inhibitors Selectively Inhibit Androgen Receptor (AR) and AR-Splice Variant (AR-SV) in Castration-Resistant Prostate Cancer (CRPC)." Cancers 15.6 (2023): 1769.
[15] Ji, Donglei, et al. "Targeting CDCP1 gene transcription coactivated by BRD4 and CBP/p300 in castration-resistant prostate cancer." Oncogene 41.23 ( 2022): 3251-3262.
[16] Alajati, A., J. Chen, and A. Alimonti. "CDCP1 initiates tumorigenesis and cooperates with PTEN loss to promote senescence evasion and prostate cancer progression." Annals of Oncology 28 (2017): v1.
[17] Chou, Chiang-Ting, et al. "Prognostic significance of CDCP1 expression in colorectal cancer and effect of its inhibition on invasion and migration." Annals of surgical oncology 22 (2015): 4335-4343.
[18] Karachaliou, Niki, et al. "Common co-activation of AXL and CDCP1 in EGFR-mutation-positive non-small cell lung cancer associated with poor prognosis. " EBioMedicine 29 (2018): 112-127.
[19] Jiang, Tao, et al. "Radiotherapy plus EGFR TKIs in non-small cell lung cancer patients with brain metastases: an update meta- analysis." Cancer Medicine 5.6 (2016): 1055-1065.
[20] Chiu, Kuo-Liang, et al. "ADAM9 enhances CDCP1 protein expression by suppressing miR-218 for lung tumor metastasis." scientific reports 5.1 (2015). 16426.
[21] Kuhara, Keisuke, et al. "CUB domain-containing protein 1 (CDCP1) is down-regulated by active hexose-correlated compound in human pancreatic cancer cells." Anticancer Research 38.11 (2018): 6107-6111.
[22] Alajati, Abdullah, et al. "Interaction of CDCP1 with HER2 enhances HER2-driven tumorigenesis and promotes trastuzumab resistance in breast cancer." Cell reports 11.4 (2015): 564-576.
[23] Cui, Yan-Hong, et al. "FBXL14 abolishes breast cancer progression by targeting CDCP1 for proteasomal degradation." Oncogene 37.43 (2018): 5794-5809.
[24] Shen, N., et al. "Effect of NLK on the proliferation and invasion of laryngeal carcinoma cells by regulating CDCP1." European Review for Medical & Pharmacological Sciences 23.14 (2019).
[25] Huang, Lijun, et al. "CUB domain-containing protein-1 promotes proliferation, migration and invasion in cervical cancer cells." Cancer Management and Research (2020): 3759-3769.
[26] Cao, Manqing, et al. "HIF-2α regulates CDCP1 to promote PKCδ-mediated migration in hepatocellular carcinoma." Tumor Biology 37 (2016): 1651-1662.
[27] Gioia, Romain, et al. "Quantitative phosphoproteomics revealed interplay between Syk and Lyn in the resistance to nilotinib in chronic myeloid leukemia cells." Blood, The Journal of the American Society of Hematology 118.8 (2011): 2211-2221.
[28] Heitmann, Jonas S., et al. "Identification of CD318 (CDCP1) as novel prognostic marker in AML." Annals of Hematology 99 (2020): 477-486.
[29] Ebian, Huda F., et al. "Evaluation of CDCP1 (CD318) and endoglin (CD105) expression as prognostic markers in acute myeloid leukemia." Cancer Biomarkers 34.2 (2022): 285-296.












