Recombinant Pseudomonas aeruginosa Azurin (azu)
-
货号:CSB-YP360518EZX
-
规格:
-
来源:Yeast
-
其他:
-
货号:CSB-EP360518EZX
-
规格:
-
来源:E.coli
-
其他:
-
货号:CSB-EP360518EZX-B
-
规格:
-
来源:E.coli
-
共轭:Avi-tag Biotinylated
E. coli biotin ligase (BirA) is highly specific in covalently attaching biotin to the 15 amino acid AviTag peptide. This recombinant protein was biotinylated in vivo by AviTag-BirA technology, which method is BriA catalyzes amide linkage between the biotin and the specific lysine of the AviTag.
-
其他:
-
货号:CSB-BP360518EZX
-
规格:
-
来源:Baculovirus
-
其他:
-
货号:CSB-MP360518EZX
-
规格:
-
来源:Mammalian cell
-
其他:
产品详情
-
纯度:>85% (SDS-PAGE)
-
基因名:azu
-
Uniprot No.:
-
别名:azu; PA4922; Azurin
-
种属:Pseudomonas aeruginosa (strain ATCC 15692 / PAO1 / 1C / PRS 101 / LMG 12228)
-
蛋白长度:full length protein
-
表达区域:21-148
-
氨基酸序列AECSVDIQGN DQMQFNTNAI TVDKSCKQFT VNLSHPGNLP KNVMGHNWVL STAADMQGVV TDGMASGLDK DYLKPDDSRV IAHTKLIGSG EKDSVTFDVS KLKEGEQYMF FCTFPGHSAL MKGTLTLK
-
蛋白标签:Tag type will be determined during the manufacturing process.
The tag type will be determined during production process. If you have specified tag type, please tell us and we will develop the specified tag preferentially. -
产品提供形式:Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
复溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
储存条件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保质期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
货期:Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事项:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet :Please contact us to get it.
靶点详情
-
功能:Transfers electrons from cytochrome c551 to cytochrome oxidase.
-
基因功能参考文献:
- Review of activity of Pseudomonas aeruginosa azurin and azurin-like bacteriocins; including hijacking of key cellular regulators and cell surface receptors to remodel the cellular signaling networks. PMID: 28960574
- this study shows that addition of Cys at position 78 modulates the functional shifting of this protein from a peroxidase to a chaperone PMID: 27457208
- Mercury metallation of the bacterial copper protein azurin is analogous to the one of human cerulopsmin and factor VIII in mercury poisoning. PMID: 25265377
- Study presents the high-resolution (1.5 A) structure of azurin from Pseudomonas aeruginosa PAO1 spin labelled with MTSSL at position T21; due to the crystal-packing environment in the triclinic crystals, the label is observed in two different but fully ordered states. PMID: 24884565
- Results suggest that a potential mechanism for the microbial toxicity of silver is the deactivation of copper oxidoreductases by the effective binding and structural mimicry by silver without the corresponding function. PMID: 23911566
- The study examines the spectral properties of tryptophan radicals in two azurin mutants (Az48W and ReAz108W). PMID: 23458492
- Atomic force spectroscopy was used to investigate the interaction of p28 peptide fragment of azurin with full-length p53 and its isolated domains at the single molecule level. PMID: 22162658
- structure of the active-site loop has a dramatic effect on the kinetic stability and the unfolding pathway PMID: 22446157
- Studies indicate that in the crystal structure of azurin the H20 is strongly hydrogen bonded to Y48 (2.7-2.8A tyrosine-O to histidine-N distance. PMID: 22210190
- Data show that almost all of the azurin variant potentials were shifted to higher values than WT azurin. PMID: 20615551
- biophysical analysis of azurin conformational changes PMID: 15345565
- Novel atomic displacement factors calculated from X-ray data from crystalline wild-type azurin indicate that residue 62 has a relative high mobility at elevated temperatures, while residue 74 is not very mobile and may act as an anchor for residue 62. PMID: 15449946
- Unfolding of the beta-barrel in azurin is associated with dislocation of its unique alpha-helix with respect to the protein scaffold. PMID: 15581373
- Pseudomonas aeruginosa azurin binds to tumor-suppressor protein p53 PMID: 15913547
- The dramatic difference in kinetic-folding behavior between apo- and zinc-forms of azurin is rationalized in terms of small changes on a common broad activation barrier PMID: 16042382
- Binding of azurin molecules to gold nanoparticle surface results in the red shift of the nanoparticle resonance plasmon band and in the quenching of the azurin single tryptophan fluorescence signal. PMID: 18938024
显示更多
收起更多
-
亚细胞定位:Periplasm.
-
数据库链接:
KEGG: pae:PA4922
STRING: 208964.PA4922
Most popular with customers
-
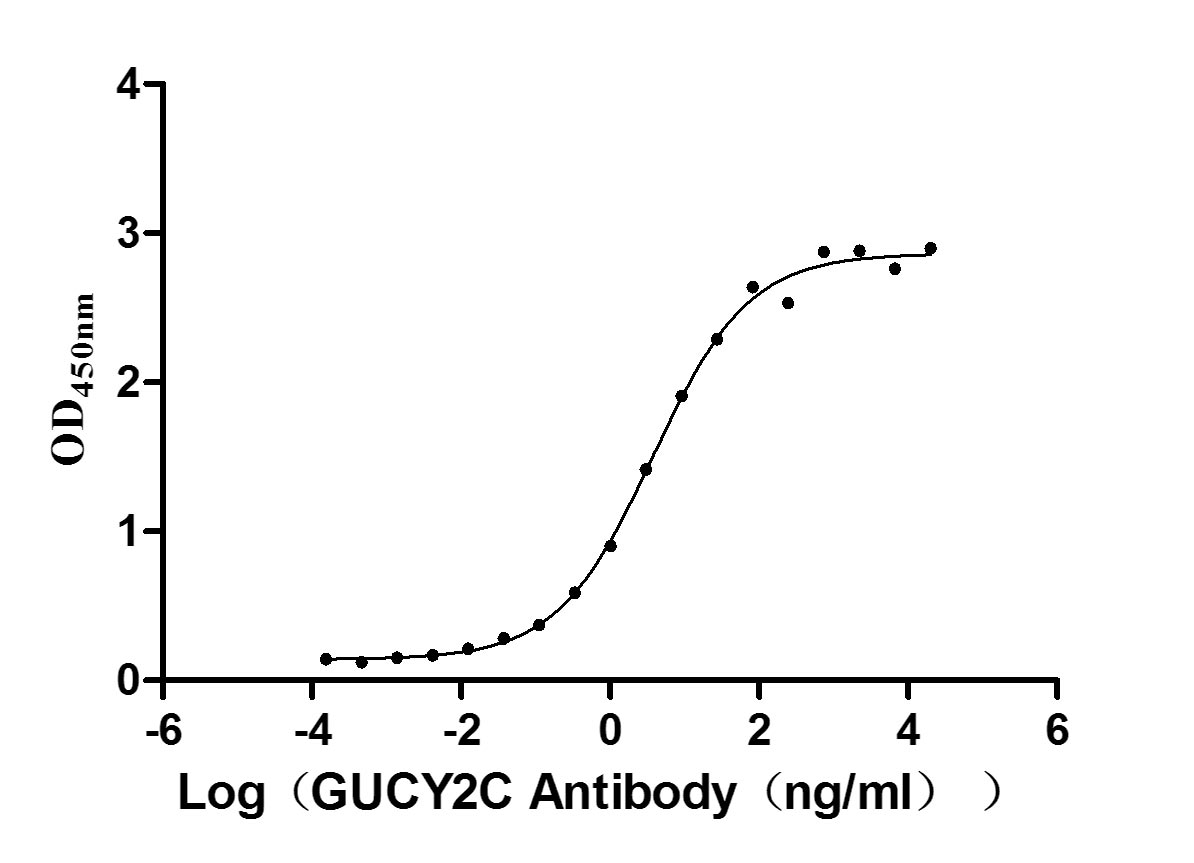
Recombinant Human Heat-stable enterotoxin receptor (GUCY2C), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
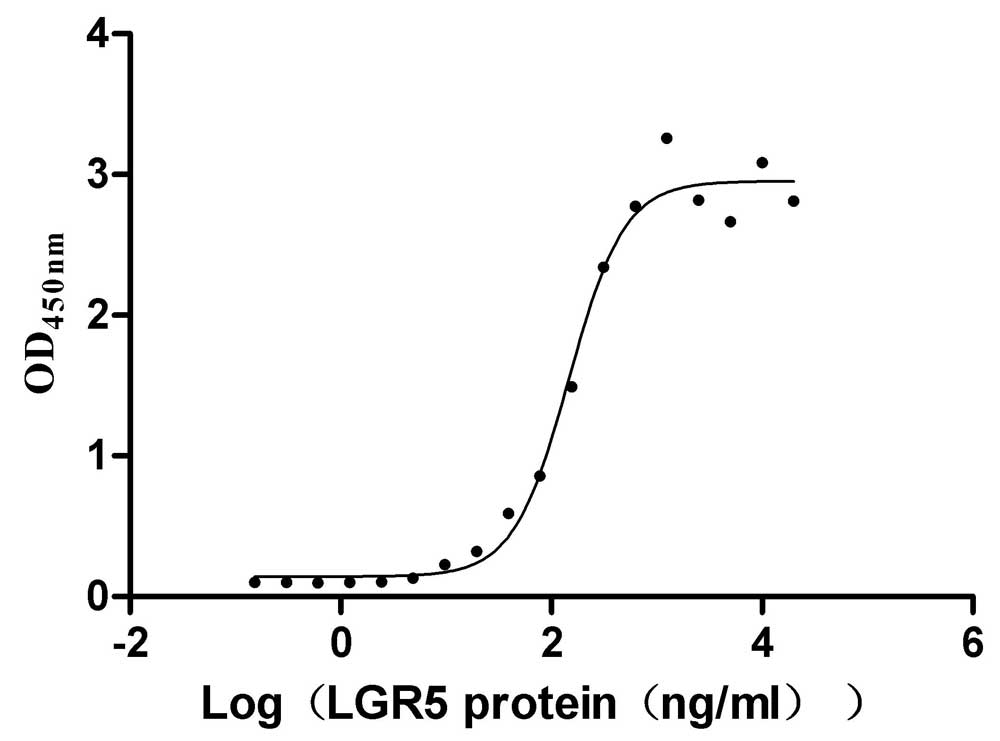
Recombinant Human R-spondin-1 (RSPO1), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
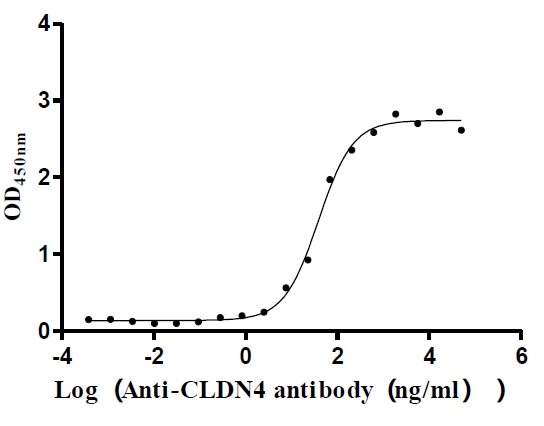
Recombinant Human Claudin-4 (CLDN4)-VLPs (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
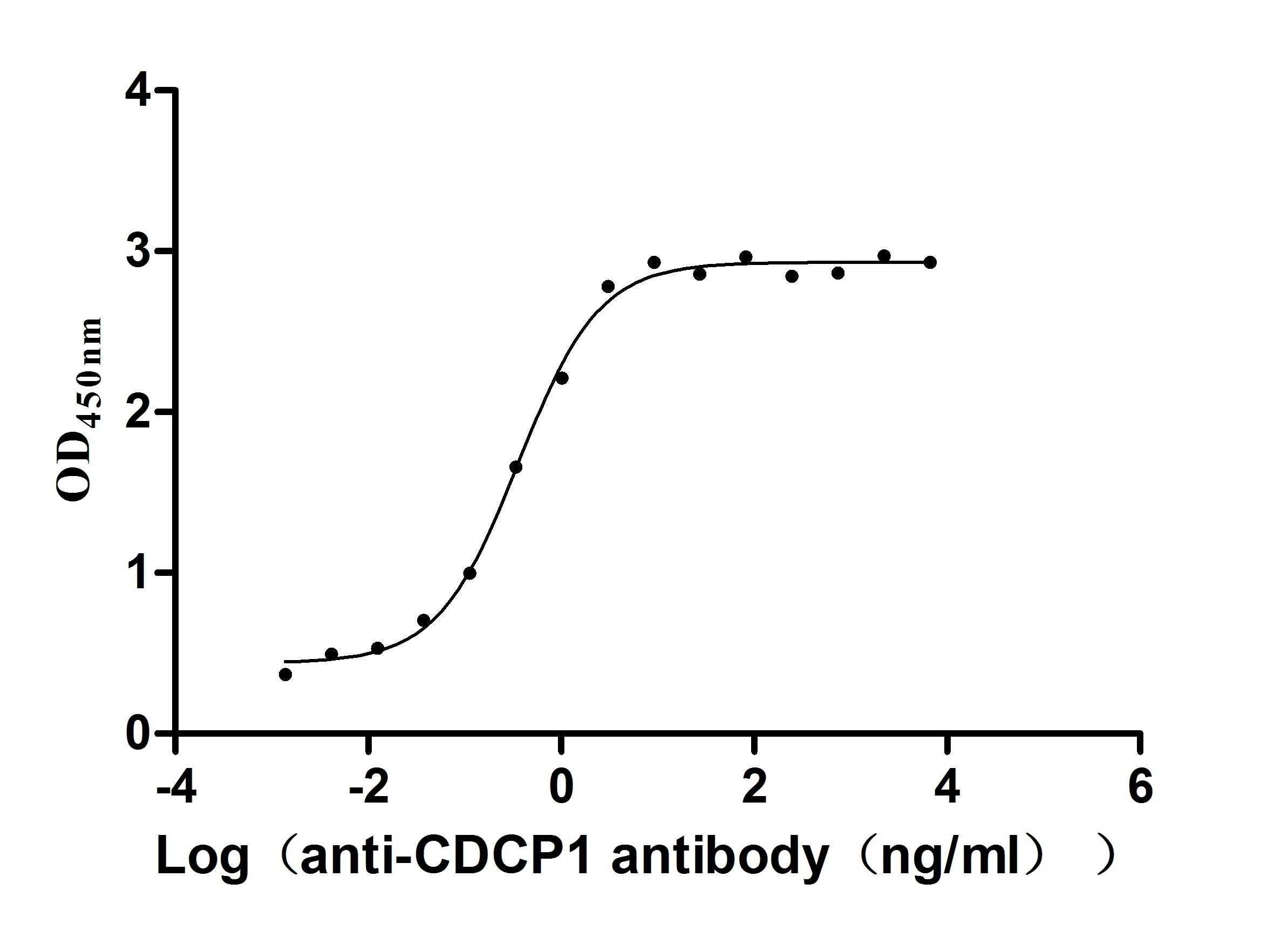
Recombinant Human CUB domain-containing protein 1 (CDCP1), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
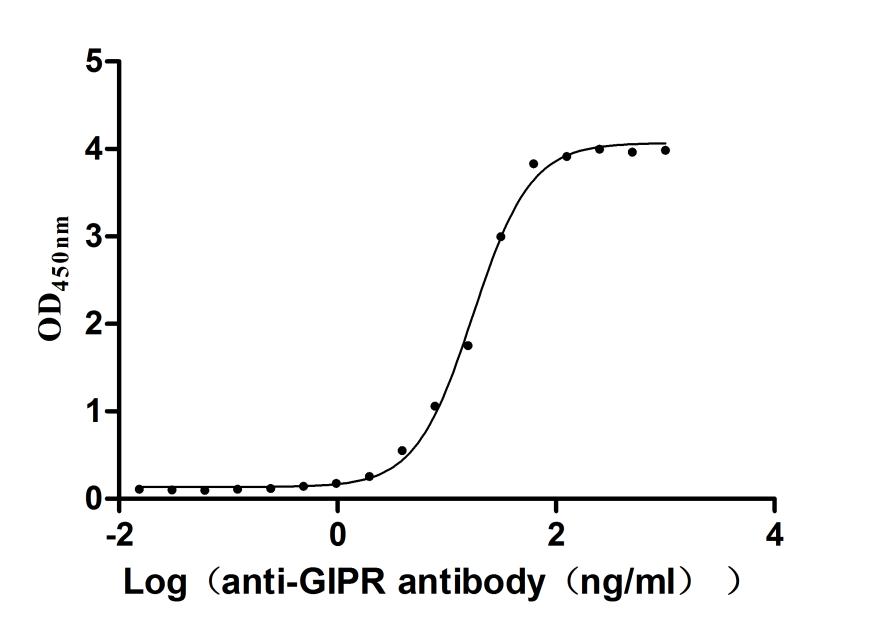
Recombinant Human Gastric inhibitory polypeptide receptor(GIPR),partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
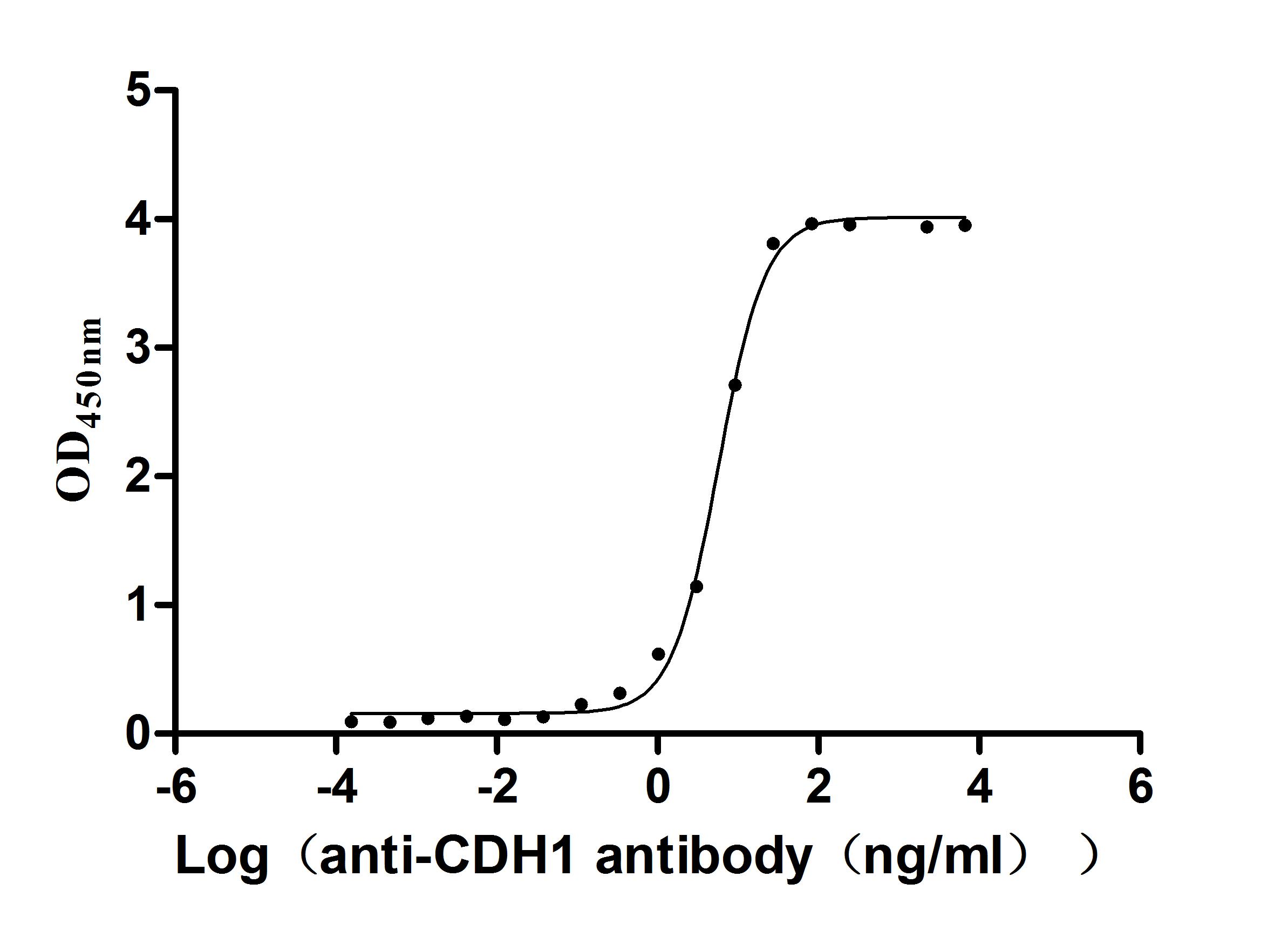
Recombinant Human Cadherin-1(CDH1),partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Urokinase-type plasminogen activator(PLAU) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
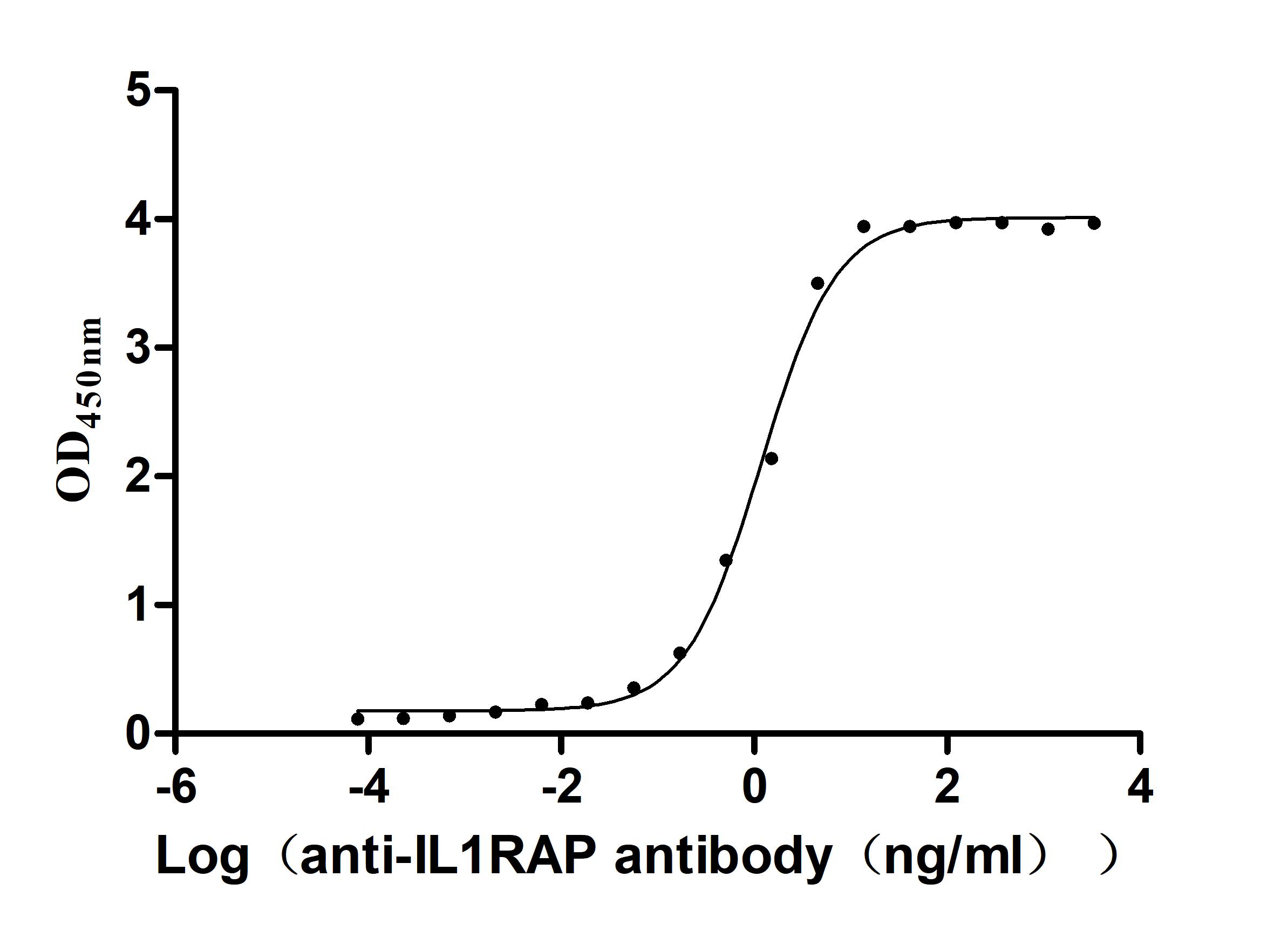
Recombinant Human Interleukin-1 receptor accessory protein (IL1RAP), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)