Recombinant Mouse Torsin-1A (Tor1a)
-
货号:CSB-YP880637MO
-
规格:
-
来源:Yeast
-
其他:
-
货号:CSB-EP880637MO
-
规格:
-
来源:E.coli
-
其他:
-
货号:CSB-EP880637MO-B
-
规格:
-
来源:E.coli
-
共轭:Avi-tag Biotinylated
E. coli biotin ligase (BirA) is highly specific in covalently attaching biotin to the 15 amino acid AviTag peptide. This recombinant protein was biotinylated in vivo by AviTag-BirA technology, which method is BriA catalyzes amide linkage between the biotin and the specific lysine of the AviTag.
-
其他:
-
货号:CSB-BP880637MO
-
规格:
-
来源:Baculovirus
-
其他:
-
货号:CSB-MP880637MO
-
规格:
-
来源:Mammalian cell
-
其他:
产品详情
-
纯度:>85% (SDS-PAGE)
-
基因名:
-
Uniprot No.:
-
别名:Tor1a; Dyt1; Torsin-1A; Dystonia 1 protein; Torsin ATPase 1; EC 3.6.4.-; Torsin family 1 member A
-
种属:Mus musculus (Mouse)
-
蛋白长度:full length protein
-
表达区域:21-333
-
氨基酸序列VEPISLSLAL AGVLTTYISY PRLYCLFAEC CGQMRSLSRE ALQKDLDNKL FGQHLAKKVI LNAVSGFLSN PKPKKPLTLS LHGWTGTGKN FASKIIAENI YEGGLNSDYV HLFVATLHFP HASNITQYKD QLQMWIRGNV SACARSIFIF DEMDKMHAGL IDAIKPFLDY YDVVDEVSYQ KAIFIFLSNA GAERITDVAL DFWKSGKQRE EIKLRDMEPA LAVSVFNNKN SGFWHSSLID RNLIDYFVPF LPLEYKHLKM CIRVEMQSRG YEVDEDIISK VAEEMTFFPK EEKVFSDKGC KTVFTKLDYY LDD
-
蛋白标签:Tag type will be determined during the manufacturing process.
The tag type will be determined during production process. If you have specified tag type, please tell us and we will develop the specified tag preferentially. -
产品提供形式:Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
复溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
储存条件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保质期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
货期:Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事项:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet :Please contact us to get it.
靶点详情
-
功能:Protein with chaperone functions important for the control of protein folding, processing, stability and localization as well as for the reduction of misfolded protein aggregates. Involved in the regulation of synaptic vesicle recycling, controls STON2 protein stability in collaboration with the COP9 signalosome complex (CSN). In the nucleus, may link the cytoskeleton with the nuclear envelope, this mechanism seems to be crucial for the control of nuclear polarity, cell movement and, specifically in neurons, nuclear envelope integrity. Participates in the cellular trafficking and may regulate the subcellular location of multipass membrane proteins such as the dopamine transporter SLC6A3, leading to the modulation of dopamine neurotransmission. In the endoplasmic reticulum, plays a role in the quality control of protein folding by increasing clearance of misfolded proteins such as SGCE variants or holding them in an intermediate state for proper refolding. May have a redundant function with TOR1B in non-neural tissues.
-
基因功能参考文献:
- TorsinA was post-transcriptionally upregulated upon acute ER stress, suggesting a role in this response. Increased basal phosphorylation of eIF2alpha in DYT1 transgenic rats was associated with abnormal response to acute ER stress. Unbiased RNA-Seq-based transcriptomic analysis of embryonic brain tissue in heterozygous and homozygous DYT1 knockin mice confirmed presence of eIF2alpha dysregulation in the DYT1 brain. PMID: 29289717
- Results show that direct pathological insult to forebrain torsinA in a symptomatic mouse model of DYT1 dystonia can engage genetically normal hindbrain regions into an aberrant connectivity network. These findings have important implications for the assignment of a causative region in CNS disease. PMID: 28673740
- that the deletion of a 3-base pair (DeltaGAG) sequence in the Dyt1 gene encoding torsinA has network level effects on in vivo functional connectivity and microstructural integrity across the sensorimotor cortex, basal ganglia, and cerebellum PMID: 27404940
- TOR1A variant found in sporadic focal dystonia impairs domains affected in DYT1 dystonia patients and animal models PMID: 27168150
- Abnormal motor symptoms in DYT1 knockdown animals were associated with irregular cerebellar output caused by changes in the intrinsic activity of both Purkinje cells and neurons of the deep cerebellar nuclei. PMID: 28198698
- Purkinje cells (PC) express high levels of TA also in the spines and axonal terminals. In addition, abundant expression of the protein was found in the main GABA-ergic and glutamatergic inputs of the cerebellar cortex. Finally, TA was observed also in glial cells, a cellular population little explored so far. These results extend our knowledge on TA synaptic localization providing a clue to its potential role in synaptic PMID: 23840813
- Study linked the genetic defect of reduced torsinA expression in a DYT1 related mouse model to a maladaptive response of the striatal dopaminergic system after a peripheral nerve lesion and to the manifestation of dystonia-like movements PMID: 27716431
- The nuclear envelope-localized AAA+ (ATPase associated with various cellular activities) torsinA (TA) and its activator, the inner nuclear membrane protein lamina-associated polypeptide 1 (LAP1), are required for rearward nuclear movement. PMID: 28242745
- This processing occurs not only in stress-exposed cell lines but also in primary cells from distinct organisms including stimulated B cells, indicating that Torsin conversion in response to physiologically relevant stimuli is an evolutionarily conserved process. PMID: 26953341
- s find no effect of this anatomic-specific expression of the DYT1 genotype. PMID: 26370418
- The data suggest that LULL1 oligomerizes to engage and transiently disassemble torsinA oligomers, and is thereby positioned to transduce cytoplasmic signals to the inner nuclear membrane through torsinA. PMID: 26092934
- These findings demonstrate that dorsal dorsal striatal large cholinergic interneurons have a unique requirement for torsinA function during striatal maturation, and link abnormalities of these cells to dystonic-like movements. PMID: 26052670
- maintaining an appropriate torsinA level is important to sustain normal motor performance, synaptic transmission and plasticity PMID: 25799505
- Dyt1 KI mice exhibit decreased striatal dopamine receptor 1 binding activity and D1R protein levels, suggesting the alteration of the direct pathway. We developed a novel motor skill transfer test for mice and found deficits in Dyt1 KI mice. PMID: 25451552
- cellular and molecular framework for how impaired torsinA function selectively disrupts neural circuits and raise the possibility that discrete foci of neurodegeneration may contribute to the pathogenesis of DYT1 dystonia PMID: 24937429
- Results reveal subtle structural changes of the cerebellum that are similar to those reported for the basal ganglia in the DYT1 knock-in mouse model. PMID: 24121114
- the mutation only slightly increases the excitability of striatal GABAergic neurons in DYT1 dystonia. PMID: 24260480
- detected differences in spontaneous locomotion between aged torsinA(DeltaE) KI-Fbg1 knock out and control mice PMID: 22917612
- The substantia nigra expression of torsinA does not protect against experimental Parkinson's disease in mice. PMID: 23185535
- Studies of the Tor1A-/- embryo show that torsinA may regulate interkinetic nuclear migration in progenitor cells & migration of newly generated neurons in the dorsal & basal forebrain. Neurogenesis is not impaired. PMID: 23018676
- These studies suggest the DYT1 mutation does not sensitize central neurons against the toxic effects of oxidative stress and energy deficits. PMID: 22880064
- ese results demonstrate a cell-autonomous effect of torsin1a deletion on striatal cholinergic function PMID: 22579992
- Mutations in torsin A gene lead to more frequent glutamate release in hippocampal neurons. PMID: 22588999
- Dyt1 Purkinje cell-specific knockout mice exhibited significantly less slip numbers in the beam-walking test, suggesting better motor performance than control littermates, and normal gait. PMID: 22391119
- Genetic background modulates the phenotype of a mouse model of DYT1 dystonia PMID: 22393392
- Results suggest that torsinA regulates synaptic vesicle recycling in central neurons and indicate that this regulation is influenced by neuronal activity, supporting the idea that synaptic abnormalities contribute to the pathophysiology of DYT1 dystonia. PMID: 22213465
- the loss of striatal torsinA alone is sufficient to produce motor deficits, and this effect may be mediated, at least in part, through changes in D2R function in the basal ganglia circuit PMID: 21931745
- The disease causing torsinA-DeltaE mutation promotes an association between torA and SUN1 that is distinct to the interaction between LAP1 and ATP-bound torA. PMID: 21627841
- These results suggest that the torsinA is important for the proper development of the cerebellum and a loss of this function in the Purkinje cells results in an alteration in dendritic structure. PMID: 21479250
- Cerebellothalamocortical pathway abnormalities in torsinA DYT1 knock-in mice. PMID: 21464304
- TorsinA is a homeostatic regulator of endoplasmic reticulum stress response. PMID: 20584926
- Wild-type Torsin A has the capacity to protect cortical neurons against oxidative stress, and in the development of DYT1-delta GAG-dystonia the neuroprotective function of wild-type Torsin A may be compromised. PMID: 20455020
- Tor1a(DeltaE/DeltaE) neural selective phenotype therefore arises because high levels of torsinB protect nonneuronal cells from the consequences of torsinA dysfunction. PMID: 20457914
- We conclude that overexpression of human wild-type or mutant torsinA does not affect the survival of adult newborn neurons. PMID: 19829161
- These results suggest that the Dyt1 DeltaGAG mutation increased the activity of the CE and enhanced the acquisition of the cued fear memory. PMID: 19619587
- TorsinA is significantly increased in the brain within hours of treatment with the dopaminergic toxin, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice, suggesting that the TorsinA gene is regulated by cellular stress. PMID: 14729251
- identifed lamina-associated polypeptide 1 (LAP1) as a torsinA-interacting protein; also identifed a novel transmembrane protein, lumenal domain like LAP1 (LULL1), which also appears to interact with torsinA PMID: 15767459
- These observations demonstrate that neurons have a unique requirement for nuclear envelope localized torsinA function and suggest that loss of this activity is a key molecular PMID: 16364897
- torsinA expression was highest in the cerebral cortex from embryonic day 15 (E15)-E17 and in the striatum from E17-P7,also highly expressed in the thalamus from P0-P7 and in the cerebellum from P7-P14. PMID: 16458269
- dyt1 knockdown mice exhibited deficits in motor control and a decreased trend in dopamine with a significant reduction in 3,4-dihydroxyphenylacetic acid PMID: 17046090
- These studies demonstrate the exquisite sensitivity of this reporter system for quantitation of processing through the secretory pathway and support a role for torsinA as an endoplasmic reticulum chaperone protein. PMID: 17428918
- Results suggest that the loss of torsinA function in the cerebral cortex alone is sufficient to induce behavioural deficits associated with Dyt1 DeltaGAG knockin mutation. PMID: 17956903
- Identification of functional proximal 5'-upstream DYT1 DNA fragments demonstrates the Ets family of proteins as critical DYT1 transcriptional regulators. PMID: 18466338
- TorsinA also associated with the KASH domains of nesprin-1 and -2 PMID: 18827015
显示更多
收起更多
-
亚细胞定位:Endoplasmic reticulum lumen. Nucleus membrane; Peripheral membrane protein. Cell projection, growth cone. Cytoplasmic vesicle membrane. Cell junction, synapse, synaptosome. Cytoplasm, cytoskeleton. Cytoplasmic vesicle, secretory vesicle. Cytoplasmic vesicle, secretory vesicle, synaptic vesicle.
-
蛋白家族:ClpA/ClpB family, Torsin subfamily
-
组织特异性:Widely expressed (at protein level).
-
数据库链接:
KEGG: mmu:30931
STRING: 10090.ENSMUSP00000028200
UniGene: Mm.154994
Most popular with customers
-
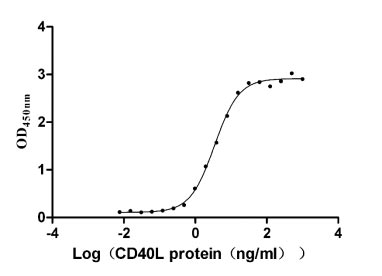
Recombinant Human Tumor necrosis factor receptor superfamily member 5 (CD40), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
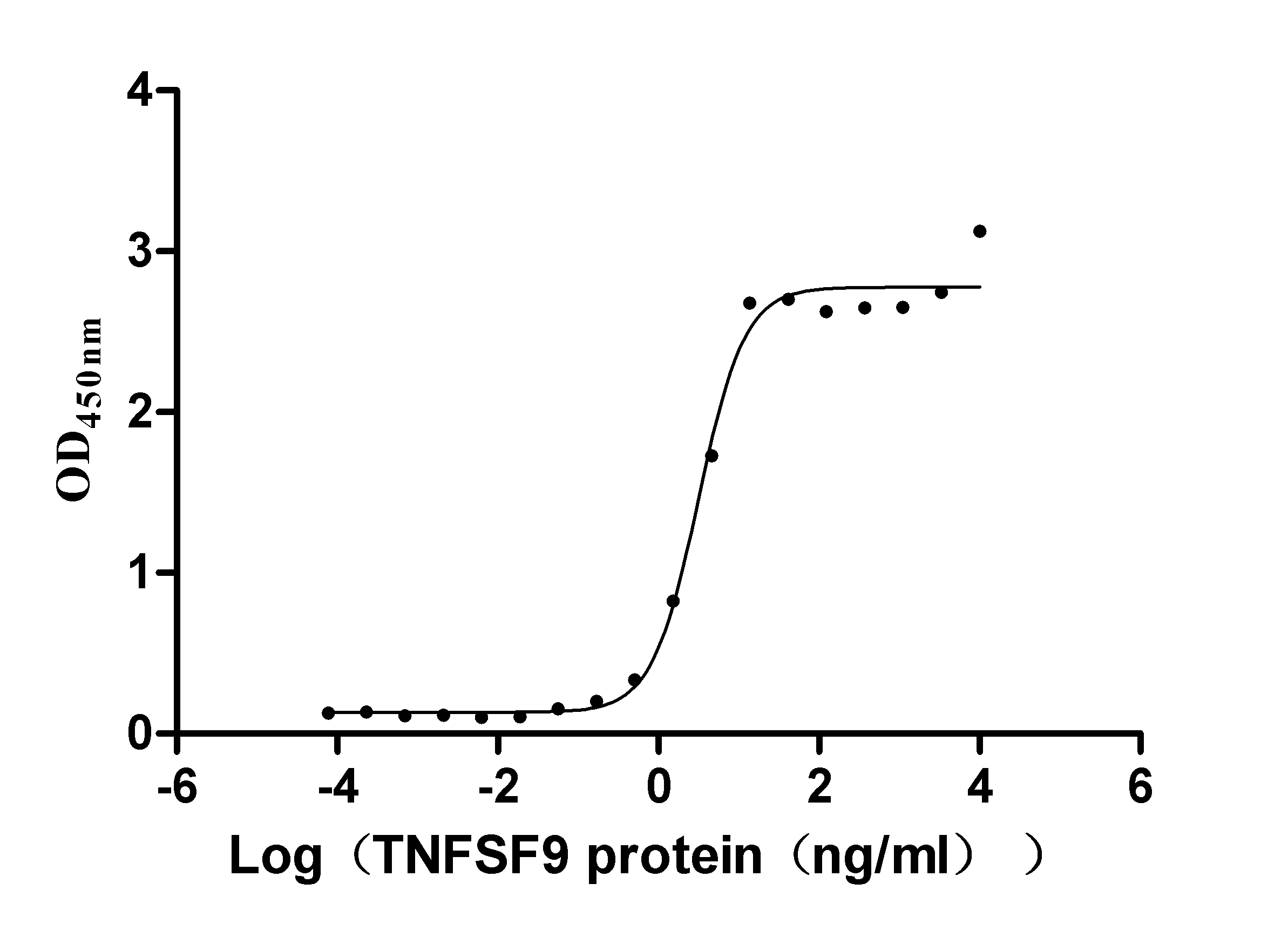
Recombinant Human Tumor necrosis factor ligand superfamily member 9 (TNFSF9), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
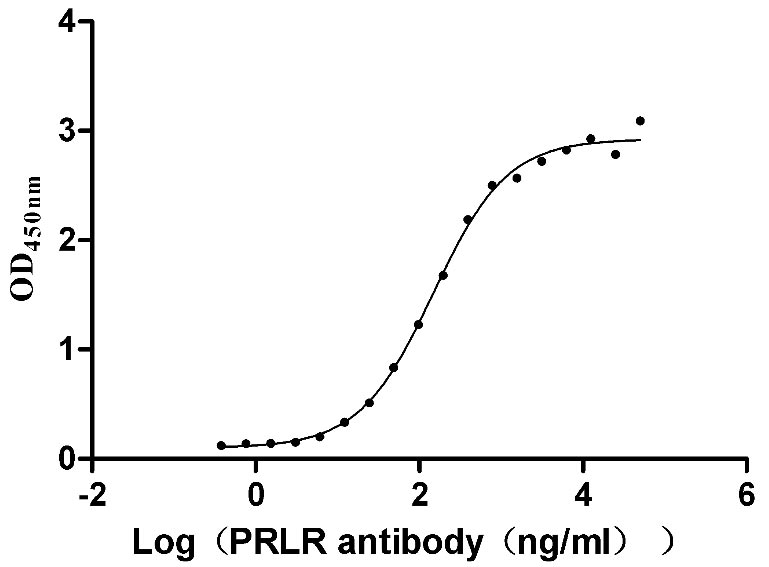
Recombinant Human Prolactin receptor (PRLR), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
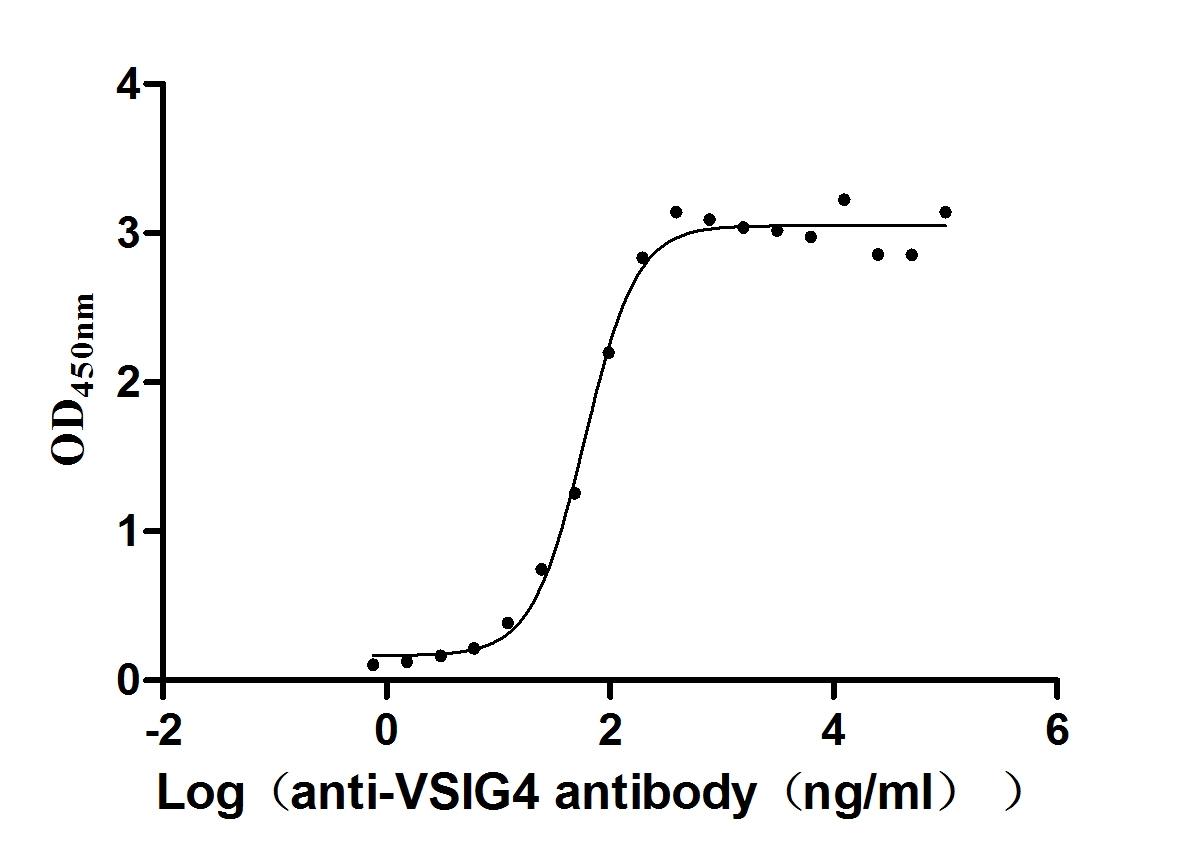
Recombinant Human V-set and immunoglobulin domain-containing protein 4 (VSIG4), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Tumor-associated calcium signal transducer 2 (TACSTD2), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
-
Recombinant Macaca fascicularis Dipeptidase 3(DPEP3) (Active)
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)
-
Recombinant Human Tumor necrosis factor ligand superfamily member 15(TNFSF15) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)


-AC1.jpg)










