Recombinant Mouse Microtubule-associated protein tau (Mapt)
In Stock-
货号:CSB-YP013481MO
-
规格:¥2208
-
图片:
-
(Tris-Glycine gel) Discontinuous SDS-PAGE (reduced) with 5% enrichment gel and 15% separation gel.
-
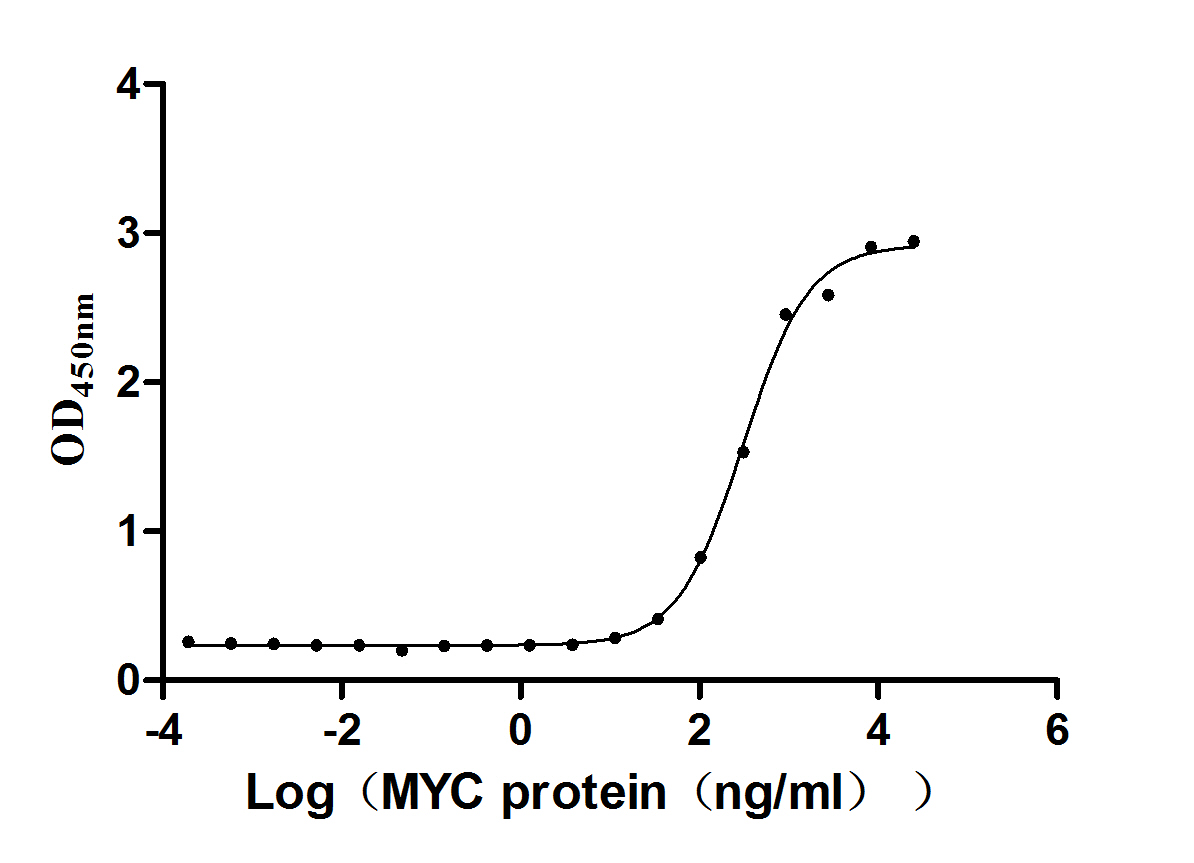
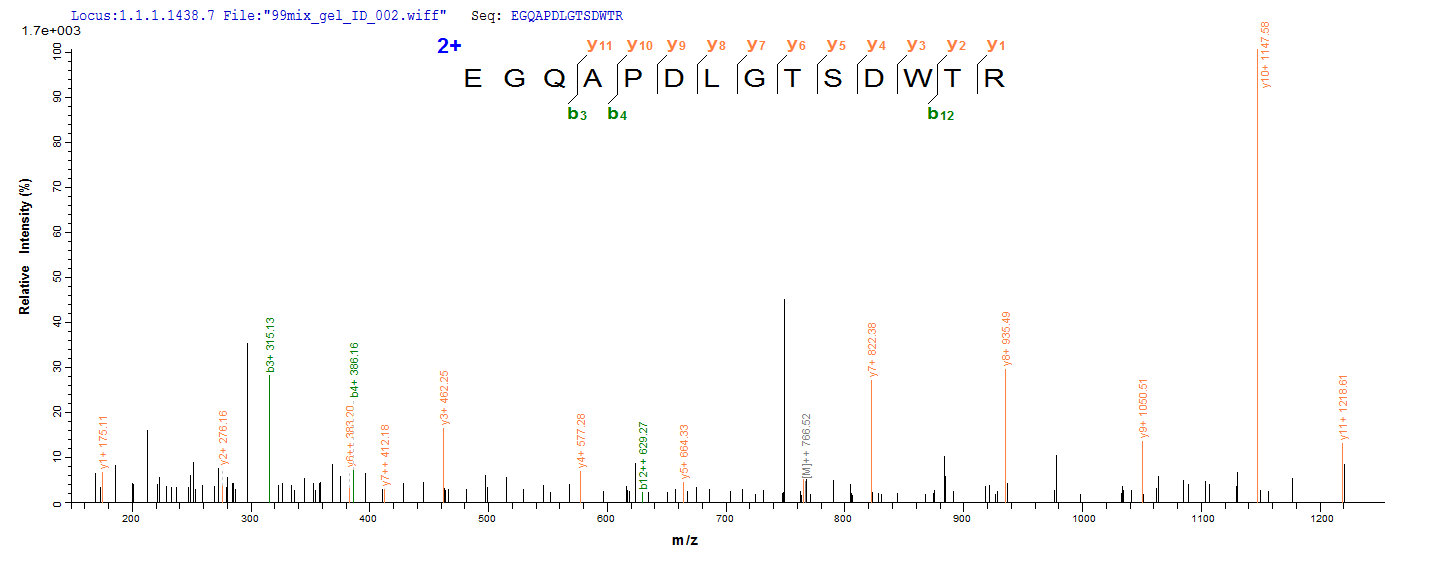
Based on the SEQUEST from database of Yeast host and target protein, the LC-MS/MS Analysis result of CSB-YP013481MO could indicate that this peptide derived from Yeast-expressed Mus musculus (Mouse) Mapt.
-
Based on the SEQUEST from database of Yeast host and target protein, the LC-MS/MS Analysis result of CSB-YP013481MO could indicate that this peptide derived from Yeast-expressed Mus musculus (Mouse) Mapt.
-
-
其他:
产品详情
-
纯度:Greater than 90% as determined by SDS-PAGE.
-
基因名:
-
Uniprot No.:
-
别名:Mapt; Mtapt; Tau; Microtubule-associated protein tau; Neurofibrillary tangle protein; Paired helical filament-tau; PHF-tau
-
种属:Mus musculus (Mouse)
-
蛋白长度:Full Length of Mature Protein
-
来源:Yeast
-
分子量:78.1kDa
-
表达区域:2-733aa
-
氨基酸序列ADPRQEFDTMEDHAGDYTLLQDQEGDMDHGLKESPPQPPADDGAEEPGSETSDAKSTPTAEDVTAPLVDERAPDKQAAAQPHTEIPEGITAEEAGIGDTPNQEDQAAGHVTQGRREGQAPDLGTSDWTRQQVSSMSGAPLLPQGLREATCQPSGTRPEDIEKSHPASELLRRGPPQKEGWGQDRLGSEEEVDEDLTVDESSQDSPPSQASLTPGRAAPQAGSGSVCGETASVPGLPTEGSVPLPADFFSKVSAETQASQPEGPGTGPMEEGHEAAPEFTFHVEIKASTPKEQDLEGATVVGVPGEEQKAQTQGPSVGKGTKEASLQEPPGKQPAAGLPGRPVSRVPQLKARVASKDRTGNDEKKAKTSTPSCAKAPSHRPCLSPTRPTLGSSDPLIKPSSPAVSPEPATSPKHVSSVTPRNGSPGTKQMKLKGADGKTGAKIATPRGAASPAQKGTSNATRIPAKTTPSPKTPPGSGEPPKSGERSGYSSPGSPGTPGSRSRTPSLPTPPTREPKKVAVVRTPPKSPSASKSRLQTAPVPMPDLKNVRSKIGSTENLKHQPGGGKVQIINKKLDLSNVQSKCGSKDNIKHVPGGGSVQIVYKPVDLSKVTSKCGSLGNIHHKPGGGQVEVKSEKLDFKDRVQSKIGSLDNITHVPGGGNKKIETHKLTFRENAKAKTDHGAEIVYKSPVVSGDTSPRHLSNVSSTGSIDMVDSPQLATLADEVSASLAKQGL
Note: The complete sequence including tag sequence, target protein sequence and linker sequence could be provided upon request. -
蛋白标签:N-terminal 6xHis-tagged
-
产品提供形式:Liquid or Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
缓冲液:Tris-based buffer,50% glycerol
-
储存条件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保质期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
货期:3-7 business days
-
注意事项:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
产品描述:
Recombinant full-length of mature Mouse Microtubule-associated protein tau (Mapt) cDNA (2-733aa) constructed with a 6xHis-tag at the N-terminus was expressed in the yeast. The protein identity was confirmed by both SDS-PAGE and LC-MS/MS analysis. It is over 90% in purity and has a calculated molecular weight of 78.1 kDa. Importantly, this recombinant Mapt protein is in stock so there is no waiting period for product preparation. The Mapt can act as an immunogen to elicit an adaptive immune reaction and thus obtain specific antibodies. Besides, this protein may also be used in the studies of the neuroscience field.
Mapt is abundant in the axons of neurons where it promotes microtubule (MT) assembly and stability. Together with stathmin and other destabilizing MAPs, Mapt also participates in MT dynamics via the modulation of assembly, dynamic behavior, and the spatial construction of MTs. Aberrant hyperphosphorylation of Mapt-causing its self-aggregation into paired helical filaments and buildup in the neurons frequently occurred in the tauopathies such as Alzheimer's disease (AD) and frontotemporal dementia (FTD).
-
Datasheet & COA:Please contact us to get it.
相关产品
问答及客户评论
We purchased mouse tau antigen product (Mouse MAPT / Tau Protein (Recombinant 6His, N-terminus, aa2-733, CSB-YP013481MO lot #03768). We tested an ELISA experiment with this product. The assay consists of two antibodies (Tau5 and BT2). An obvious signal was found when human tau (2N4R) is used as an antigen, but no signal was found when CSB-YP013481MO is used as an antigen. These two tests were performed on the same plate and same time. The epitope of Tau5 is aa504-511 (PPTREPKK) of L CSB-YP013481MO and the epitope of BT2 is aa480-484 (RSGYS) of CSB-YP013481MO. Both epitopes are exist in human tau and mouse tau. We wonder why mouse antigen CSB-YP013481MO gave no signal on this ELISA. It has questioned the certainty of the product.
1. The Tau monoclonal antibody (Tau5 and BT2) you used should be one of the following:
https://www.thermofisher.com/antibody/product/Tau-Antibody-clone-TAU-5-Monoclonal/MA5-12808
https://www.thermofisher.com/antibody/product/Tau-Antibody-clone-TAU-5-Monoclonal/AHB0042
https://www.novusbio.com/products/tau-antibody-tau-5_nb200-514
https://www.thermofisher.com/antibody/product/Tau-Antibody-clone-BT2-Monoclonal/MN1010
From your WB test result: Both of Tau-5 and BT2 recognize the 50kDa band. Tau has 9 isomers, and its standard isomer is Isoform PNS-tau with calculated value of 78.9kDa, which has multiple post-translation modifications (glycosylation/phosphorylation/acetylation). And its observed size must be larger than 80 kDa. This shows that neither of these two antibodies recognize the standard isoform (Isoform PNS-tau).
2. You used Tau (2N4R) as an antigen and the signal was detected. The corresponding information is as below: https://www.rpeptide.com/products/proteins/tau/t-1001-1
It corresponds to the tau-4 isomer (Isoform Tau- F) and its calculated value is 45.8 kDa. Taking the post-translational modification into consideration, its observed size should larger than 45.8 kDa. Combined with the previous two mAb information (Both recognized ~50 kDa band), it is presumed that both of these two mAbs recognize tau-4 isomer (identifier: P10636-8).
3. What we provided was the standard isoform of mouse species: Isoform PNS-Tau (identifier: P10637-1) with a calculated value of 76.2 kDa.
The two monoclonal antibodies did not recognize the standard isoform of human species: Isoform PNS-tau (identifier: P10636-1 ). It is likely that the standard isomers of mouse also can not recognized. The structure of different isomers is not exactly the same, and it is likely that the epitopes recognized by these two mAbs are different in the structure of standard isomers and tau-4 isomers
(existence of entrapment/non-linearity, etc.).
4. We have performed Mass Spectrometry Identification for this protein (CSB-YP013481MO) and have uploaded the MS image onto our website. You could have a look. Here is the link: https://www.cusabio.com/Recombinant-Protein/Recombinant-Mouse-Microtubule-associated-protein-tauMapt-3547.html
5. If you want the mouse antibody against this protein, we can provide customize service for this antibody with preferential price.
靶点详情
-
功能:Promotes microtubule assembly and stability, and might be involved in the establishment and maintenance of neuronal polarity. The C-terminus binds axonal microtubules while the N-terminus binds neural plasma membrane components, suggesting that tau functions as a linker protein between both. Axonal polarity is predetermined by tau localization (in the neuronal cell) in the domain of the cell body defined by the centrosome. The short isoforms allow plasticity of the cytoskeleton whereas the longer isoforms may preferentially play a role in its stabilization.
-
基因功能参考文献:
- that tau modulates motility in a motor-specific manner to direct intracellular transport PMID: 29077261
- the AD-like tau accumulation induces anxiety through disrupting miR92a-vGAT-GABA signaling, which reveals molecular mechanisms underlying the anxiety behavior in AD patients and potentially leads to the development of new therapeutics for tauopathies. PMID: 28129110
- These results show for the first time that the phosphorylation and isoform alteration of tau are regulated differently during mouse development. PMID: 29196605
- In adult and aged tau(+/+), tau(+/-), tau(-/-) mice tau deficiency could not induce significant motor disorders. However, found lower expression levels of transcription factors Orthodenticle homeobox 2 (OTX2) of midbrain dopaminergic neurons in older aged mice. Results suggested that tau deficiency alone might not be enough to mimic the pathology of Parkinson's disease. PMID: 29337233
- Tau deletion increased ATP production and improved the recognition memory and attentive capacity of juvenile mice. PMID: 30077079
- MAPT variant interaction with mutant amyloid protein precursor causes frontotemporal dementia. PMID: 29729423
- These results establish that in addition to the neuritic plaque, a second determinant is required to drive the conversion of wild-type tau. PMID: 27373369
- miR-322 promotes Tau phosphorylation via negatively controlling BDNF-TrkB receptor activation PMID: 29464486
- High tau expression is associated with blood vessel abnormalities and angiogenesis in Alzheimer's disease. PMID: 29358399
- Disinhibiation of Gas6 binding to Tyro3 due to PGRN reduction results in activation of PKCalpha via PLCgamma, inducing tau phosphorylation at Ser203, mislocalization of tau to dendritic spines, and spine loss. PMID: 29382817
- frontotemporal dementia and parkinsonism linked to chromosome 17 tau with a mutation in the C-terminal region had different banding patterns, indicating a different phosphorylation pattern. PMID: 27641626
- For the three conditions, FRAP analysis revealed a similar mobility in dendrites compared with axons; however, Tau-mEOS2 was less mobile than hWT-Tau and hP301L-Tau and the mobile fraction was smaller, possibly reflecting less efficient microtubule binding of Tau when over-expressed. Together, our study presents Tau-mEOS2 mice as a novel tool for the study of Tau in a physiological and a pathological context. PMID: 27378256
- Chronic Dyrk1 inhibition reversed cognitive deficits in Alzheimer's disease transgenic mice via reduction of APP and phosphorylated tau pathology. PMID: 28779511
- Results suggest the importance of the autophagosome for the low-frequency stimulation-induced oligomerization of tau and suggest a reason for its age dependency. Interestingly, the lysosomal disturbance promoted the formation of the fibrillar form of aggregates consisting of hyper-phosphorylated tau. PMID: 28874186
- deletion or inhibition of the cytoplasmic shuttling factor HDAC6 suppressed neuritic tau bead formation in neurons. PMID: 28854366
- The present findings support the idea that the progressive accumulation of phospho-tau is associated with structural alterations of the GA, and that these changes may occur in the absence of Abeta pathology. PMID: 28922155
- MicroRNA-146a suppresses ROCK1 allowing hyperphosphorylation of tau in Alzheimer's disease. PMID: 27221467
- results demonstrate that ApoE affects tau pathogenesis, neuroinflammation, and tau-mediated neurodegeneration independently of amyloid-beta pathology; ApoE4 exerts a 'toxic' gain of function whereas the absence of ApoE is protective PMID: 28959956
- Study demonstrated that, in ob/ob mice, type 2 diabetes leads to a progressive tau hyperphosphorylation due to hypothermia, which is reversed by normothermia but not by acute leptin injections or 15 weeks caffeine treatment. PMID: 27793638
- study reports that tau is present in the heart and loss of tau in the heart causes elevated blood pressure and altered cardiac performance in aged mice. PMID: 28059795
- These data are consistent with other observations that the rapidly depositing Tg4510 mouse is a challenging model in which to demonstrate efficacy of tau-lowering treatments compared to some other preclinical models of tau deposition/overexpression. PMID: 28655349
- Data show that tau promotes excitotoxicity by a post-synaptic mechanism. PMID: 28883427
- Tau as an essential mediator of the adverse effects of stress on brain structure and function. PMID: 27274066
- Study demonstrated that phosphorylated-tau spreads gradually and selectively from the injured cortex to other brain regions after traumatic brain injury and that all of the affected regions are part of the working memory circuit. PMID: 28163095
- Study demonstrates a mechanistic link between brain Abeta deposition and CSF tau, and thus, CSF tau may present an important readout of Abeta deposition in mouse models and likely in Alzheimer's disease. PMID: 27750032
- Tau efflux from the brain to the blood was evaluated by administering radioactively labeled and unlabeled tau intracerebroventricularly in wild-type and tau knock-out mice, respectively. The efflux of Tau, including a fraction via CNS-derived L1CAM exosomes, was observed in mice. Tau is readily transported from the brain to the blood. PMID: 27234211
- Somatodendritic accumulation of Tau in Alzheimer's disease is promoted by Fyn-mediated local protein translation. PMID: 28864542
- Intermittent hypoxia treatment (IHT)-induced cognitive impairment may be partially explained by the fact that IHT increases phosphorylated tau via biological processes common to aging. PMID: 28057021
- Pin1 serves as a positive regulatory molecule of proplatelet formation of megakaryocytes by enhancing the function of phosphorylated tau. PMID: 28943044
- PGRN decrease, resulting from pathogenic mutations, might compromise the trophism of cortical neurons by affecting GluN2B-contaning NMDA receptors PMID: 28899992
- These data provide evidence that amyloid beta acts to enhance tau pathology by increasing the formation of tau species capable of seeding new aggregates. PMID: 28500862
- These results suggest that tau haploinsufficiency, without the compensation effect of MAP1A, induces reduction of Otx2 expression, increases prenatal cell death, and accordingly leads to selective loss of VTA DA neurons in the early postnatal stage. PMID: 28424350
- High-glucose induces tau hyperphosphorylation through activation of TLR9-P38 MAPK pathway. PMID: 28803064
- these results uncover a novel role for mDia1 in Abeta-mediated synaptotoxicity and demonstrate that inhibition of MT dynamics and accumulation of PTMs are driving factors for the induction of tau-mediated neuronal damage. PMID: 28877993
- The s show here that miR-132 loss exacerbates both amyloid and TAU pathology via inositol 1,4,5-trisphosphate 3-kinase B (ITPKB) upregulation in an Alzheimer's disease mouse model. PMID: 27485122
- TIA1 knockdown or knockout inhibits tau misfolding and associated toxicity in cultured hippocampal neurons, while overexpressing TIA1 induces tau misfolding and stimulates neurodegeneration. PMID: 27160897
- Data suggest that a switch in post-translational processing of Tau from acetylation at Lys321 to phosphorylation at Ser324 coordinately regulates Tau aggregation and may be relevant in tauopathy and Alzheimer disease; acetylation/phosphorylation of Tau appears to be controlled by Hdac6 (histone deacetylase 6 protein). PMID: 28760828
- ROS produced by 1,2-diacetylbenzene causes tau hyperphosphorylation via GSK-3beta phosphorylation and it might be related to impaired memory deficit. PMID: 28734998
- tau overexpression mediates the excitatory toxicity induced by E-NMDAR activation through inhibiting ERK phosphorylation. PMID: 27809304
- Data suggest that a presumed diffusion barrier within axon initial segment (AIS) regulates wild-type Tau sorting: retrograde (axon-to-soma) and anterograde (soma-to-axon) sorting of Tau. Tau isoforms without N-terminal inserts are sorted efficiently into axons; a longer isoform (2N4R-Tau) is partially retained in cell bodies/dendrites and accelerates spine/dendrite growth. PMID: 28536263
- This study establishes a mouse model of sporadic tauopathies and points to important differences between tau fibrils that are generated artificially and authentic ones that develop in Alzheimer's disease brains. PMID: 27810929
- tau diffusion on microtubules enables to keep microtubules evenly distributed in axonal sections at low tau levels. PMID: 27076215
- Upregulating HSF1 relieves the tau toxicity in N2a-TauRD DeltaK280 by reducing CHOP and increasing HSP70 a5 (BiP/GRP78). Our work reveals how the bidirectional crosstalk between the two stress response systems promotes early tau pathology and identifies HSF1 being one likely key player in both systems. PMID: 28678786
- our findings suggest that tau is a common downstream factor in both amyloid-dependent and-independent pathogenic mechanisms and therefore could be a more effective drug target for therapeutic intervention in AD. PMID: 27459671
- Study focused functional consequences of human tau accumulation: aging htau mice (deficient of murine tau but express all six human tau isoforms) compared with murine tau knock-out and C57Bl/6J mice; reduced food-burrowing performance was the most robust aspect of the htau phenotype with aging, htau phenotype appeared stronger than the mtau(-/-) phenotype at young ages but milder with aging. PMID: 27167086
- Results may provide support for the hypothesis that enhanced expression of tau following lipopolysaccharide administration is a protective measure by hippocampal neurons to compensate for the loss of the microtubule-stabilizing protein due to phosphorylation. More importantly, our results support the hypothesis that blocking the production of Abeta that follows inflammation also leads to reduced tau phosphorylation PMID: 27320209
- Study identified microtubule-associated protein tau as a highly sensitive constituent of the cytoskeleton in the presence of experimental stroke, thus providing novel evidence for a pivotal role of cytoskeletal elements under ischemic conditions PMID: 27189884
- These findings suggest that TDP-43 promotes tau exon 10 inclusion and 4R-tau expression and that disease-related changes of TDP-43, truncations and mutations, affect its function in tau exon 10 splicing, possibly because of TDP-43 mislocalization to the cytoplasm. PMID: 28487370
- Thus, these data demonstrate that Tau(-/-) mice show impairments in the maturation of newborn granule neurons under basal conditions and that they are insensitive to the modulation of adult hippocampal neurogenesis exerted by both stimulatory and detrimental stimuli. PMID: 27198172
- this work provides insights into postsynaptic processes in Alzheimer's disease pathogenesis and challenges a purely pathogenic role of tau phosphorylation in neuronal toxicity. PMID: 27856911
显示更多
收起更多
-
相关疾病:May be involved in the pathogenesis of cytoplasmic inclusions (as Mallory bodies) in livers of mice chronically intoxicated with Griseofulvin or DDC (3,5-diethoxycarbonyl-2,4-dihydrocollidine), a model for human alcoholic hepatitis. Alteration of Tau (abnormal phosphorylation and cross-linking) could contribute to Mallory bodies formation and disturbance of microtubule function in alcoholic liver disease.
-
亚细胞定位:Cytoplasm, cytosol. Cell membrane; Peripheral membrane protein; Cytoplasmic side. Cytoplasm, cytoskeleton. Cell projection, axon. Cell projection, dendrite. Secreted.
-
组织特异性:Expressed in neurons and at a lower level in the liver and kidney. Isoform PNS-tau is expressed in the peripheral nervous system while the others are expressed in the central nervous system.
-
数据库链接:
KEGG: mmu:17762
STRING: 10090.ENSMUSP00000097919
UniGene: Mm.1287
Most popular with customers
-
Recombinant Human Glypican-3 (GPC3) (G537R), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human papillomavirus type 16 Protein E7 (E7) (Active)
Express system: E.coli
Species: Human papillomavirus type 16
-
Recombinant Human E3 ubiquitin-protein ligase ZNRF3 (ZNRF3), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Macaca fascicularis CD44 antigen (CD44), partial (Active)
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)
-
Recombinant Human V-set and immunoglobulin domain-containing protein 4 (VSIG4), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Cell adhesion molecule 1 (CADM1), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Macaca fascicularis CUB domain containing protein 1 (CDCP1), partial (Active)
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)
-
Recombinant Human Kidney-associated antigen 1(KAAG1) (Active)
Express system: Baculovirus
Species: Homo sapiens (Human)




-AC1.jpg)