Recombinant Mouse Heat shock protein HSP 90-beta (Hsp90ab1)
-
货号:CSB-YP010808MO
-
规格:
-
来源:Yeast
-
其他:
-
货号:CSB-EP010808MO
-
规格:
-
来源:E.coli
-
其他:
-
货号:CSB-EP010808MO-B
-
规格:
-
来源:E.coli
-
共轭:Avi-tag Biotinylated
E. coli biotin ligase (BirA) is highly specific in covalently attaching biotin to the 15 amino acid AviTag peptide. This recombinant protein was biotinylated in vivo by AviTag-BirA technology, which method is BriA catalyzes amide linkage between the biotin and the specific lysine of the AviTag.
-
其他:
-
货号:CSB-BP010808MO
-
规格:
-
来源:Baculovirus
-
其他:
-
货号:CSB-MP010808MO
-
规格:
-
来源:Mammalian cell
-
其他:
产品详情
-
纯度:>85% (SDS-PAGE)
-
基因名:
-
Uniprot No.:
-
别名:Hsp90ab1; Hsp84; Hsp84-1; Hspcb; Heat shock protein HSP 90-beta; Heat shock 84 kDa; HSP 84; HSP84; Tumor-specific transplantation 84 kDa antigen; TSTA
-
种属:Mus musculus (Mouse)
-
蛋白长度:Full Length of Mature Protein
-
表达区域:2-724
-
氨基酸序列PEEVHHGEE EVETFAFQAE IAQLMSLIIN TFYSNKEIFL RELISNASDA LDKIRYESLT DPSKLDSGKE LKIDIIPNPQ ERTLTLVDTG IGMTKADLIN NLGTIAKSGT KAFMEALQAG ADISMIGQFG VGFYSAYLVA EKVVVITKHN DDEQYAWESS AGGSFTVRAD HGEPIGRGTK VILHLKEDQT EYLEERRVKE VVKKHSQFIG YPITLYLEKE REKEISDDEA EEEKGEKEEE DKEDEEKPKI EDVGSDEEDD SGKDKKKKTK KIKEKYIDQE ELNKTKPIWT RNPDDITQEE YGEFYKSLTN DWEDHLAVKH FSVEGQLEFR ALLFIPRRAP FDLFENKKKK NNIKLYVRRV FIMDSCDELI PEYLNFIRGV VDSEDLPLNI SREMLQQSKI LKVIRKNIVK KCLELFSELA EDKENYKKFY EAFSKNLKLG IHEDSTNRRR LSELLRYHTS QSGDEMTSLS EYVSRMKETQ KSIYYITGES KEQVANSAFV ERVRKRGFEV VYMTEPIDEY CVQQLKEFDG KSLVSVTKEG LELPEDEEEK KKMEESKAKF ENLCKLMKEI LDKKVEKVTI SNRLVSSPCC IVTSTYGWTA NMERIMKAQA LRDNSTMGYM MAKKHLEINP DHPIVETLRQ KAEADKNDKA VKDLVVLLFE TALLSSGFSL EDPQTHSNRI YRMIKLGLGI DEDEVTAEEP SAAVPDEIPP LEGDEDASRM EEVD
-
蛋白标签:Tag type will be determined during the manufacturing process.
The tag type will be determined during production process. If you have specified tag type, please tell us and we will develop the specified tag preferentially. -
产品提供形式:Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
复溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
储存条件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保质期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
货期:Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事项:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet :Please contact us to get it.
相关产品
靶点详情
-
功能:Molecular chaperone that promotes the maturation, structural maintenance and proper regulation of specific target proteins involved for instance in cell cycle control and signal transduction. Undergoes a functional cycle linked to its ATPase activity. This cycle probably induces conformational changes in the client proteins, thereby causing their activation. Interacts dynamically with various co-chaperones that modulate its substrate recognition, ATPase cycle and chaperone function. Engages with a range of client protein classes via its interaction with various co-chaperone proteins or complexes, that act as adapters, simultaneously able to interact with the specific client and the central chaperone itself. Recruitment of ATP and co-chaperone followed by client protein forms a functional chaperone. After the completion of the chaperoning process, properly folded client protein and co-chaperone leave HSP90 in an ADP-bound partially open conformation and finally, ADP is released from HSP90 which acquires an open conformation for the next cycle. Apart from its chaperone activity, it also plays a role in the regulation of the transcription machinery. HSP90 and its co-chaperones modulate transcription at least at three different levels. They first alter the steady-state levels of certain transcription factors in response to various physiological cues. Second, they modulate the activity of certain epigenetic modifiers, such as histone deacetylases or DNA methyl transferases, and thereby respond to the change in the environment. Third, they participate in the eviction of histones from the promoter region of certain genes and thereby turn on gene expression. Antagonizes STUB1-mediated inhibition of TGF-beta signaling via inhibition of STUB1-mediated SMAD3 ubiquitination and degradation. Promotes cell differentiation by chaperoning BIRC2 and thereby protecting from auto-ubiquitination and degradation by the proteasomal machinery. Main chaperone involved in the phosphorylation/activation of the STAT1 by chaperoning both JAK2 and PRKCE under heat shock and in turn, activates its own transcription. Involved in the translocation into ERGIC (endoplasmic reticulum-Golgi intermediate compartment) of leaderless cargos (lacking the secretion signal sequence) such as the interleukin 1/IL-1; the translocation process is mediated by the cargo receptor TMED10.
-
基因功能参考文献:
- The results demonstrate that in renal cells, NHE1 is associated with several regulatory proteins including Hsp90, and that Hsp90 affects its function possibly through altered phosphorylation of the protein via the AKT kinase. PMID: 28483634
- Hsp90 is highly expressed on the cell surface of melanoma cells, and synthetic agents that target Hsp90 are promising cancer therapeutic drugs PMID: 27575453
- Data show that docetaxel, rapamycin and tanespimycin multi-drug loaded micelles targeted against HSP90 and the PI3K/AKT/mTOR pathway in prostate cancer. PMID: 28350865
- genes respond to HSP90 inhibition in a manner dependent on their genomic location with regard to strain-specific endogenous retroviruses-insertion sites. PMID: 28134929
- PABPN1 interacts with and is stabilized by heat shock protein 90. PMID: 26414348
- These findings demonstrate a critical role of Hsp90 in lipopolysaccharide (LPS) signaling, and a potential involvement of the heat shock response in LPS-induced preconditioning. PMID: 24646925
- Phosphoproteomics, protein expression inference and signaling pathway prediction analysis of P2RX7 signaling mediators pointed to HSPA2 and HSP90 proteins. PMID: 25700737
- Hsp90 inhibition plays a key role in preventing the recurrence of HCC, and the combination of ablation with targeted therapy holds great potential to improve prognosis and survival of HCC patients. PMID: 25403416
- These findings demonstrate CKII induces polymerization of soluble TDP-43 into filaments and Hsp90 promotes TDP-43 filament depolymerization. PMID: 24595055
- Reveal an opposite role of Hsp70 and Hsp90 in regulating TGF-beta signaling by implicating CHIP-mediated Smad3 ubiquitination and degradation. This study provides a new insight into understanding the regulation of the TGF-beta signaling by chaperones. PMID: 24613385
- A novel role for Hsp90 in controlling PPARgamma stability and cellular differentiation, is reported. PMID: 24096869
- inhibition exerts an anti-inflammatory effect in murine models of colitis PMID: 23321985
- Hsp90 activity is necessary to control the expression, activity or location of specific kinases and motor proteins during the axon specification and axon elongation processes. PMID: 24286867
- Alcohol-induced cilia stimulation occurs through the increased association of HSP90 with miR-122 target gene endothelial nitric oxide synthase (eNOS). PMID: 23078267
- Results suggest that heat shock protein 90 inhibitor radicicol inhibited 3T3-L1 preadipocyte differentiation through affecting the PDK1/Akt pathway. PMID: 23727383
- Perturbation of the HSP70-HSP90 heat-shock protein axis stimulates degradation of endothelial VEGFR2. PMID: 23139789
- Hsp90 expression is up-regulated in injured arteries and colocalizes with the apoptosis inhibitor, survivin, in vascular smooth muscle cell in vitro and in vivo. PMID: 22841823
- HSP90 is expressed in the vast majority of pancreatic endocrine tumors and inhibition of HSP90 results in a significant reduction of cell viability, cell cycle arrest, and increased apoptosis PMID: 22194440
- HSP90 may modulate myogenic differentiation and may be involved in cell survival. PMID: 21739150
- This study concluded that hsp90 plays a primary role in maturation of iNOS protein by interacting with the apoenzyme in cells and then driving heme insertion in an ATP-dependent manner. PMID: 21357526
- involved in translocation of a chaperoned antigen from endosome to proteasome for cross-presentation by dendritic cells PMID: 21421737
- The beneficial effects of HDAC6 targeting are also achieved by inhibition of the HDAC6-regulated protein heat shock protein 90. Inhibition of HSP90 enhances the suppressive functions of Foxp3+ Tregs. PMID: 21444725
- This results in the transcriptional repression of Hsp90beta, under GSH-deficient conditions which may play a role in arsenic-mediated pathogenesis. PMID: 18996350
- Heat shock protein 84 from C57BL/6 mice modulates higher cellular glucocorticoid-glucocorticoid receptor responsiveness. PMID: 20332616
- This study is the first report identifying four genetic variations of the murine hsp84 gene: 226A>C, 996G>C, 1483G>C, and 2000G>T. PMID: 19845467
- Data show that inhibition of Hsp90 engages a p53-dependent pathway to apoptosis. PMID: 19805107
- Hsp90 binding to Galpha12 helps target Galpha12 to lipid rafts PMID: 12117999
- peroxisome proliferator-activated receptor alpha is complexed with the 90-kDa heat shock protein PMID: 12482853
- mineralocorticoid receptor and HSP84 mediate Src activation by aldosterone PMID: 15251441
- in the cytoplasm linker histones are bound to a complex containing NASP and HSP90 whose ATPase activity is stimulated by binding NASP PMID: 15533935
- The antiapoptotic activity of Bcl-2 was greatly affected by knocking-out specifically Hsp90beta using the RNA interference approach. PMID: 16166581
- Hsp90beta is distinct from Hsp90alpha in regulation of the cellular function of immune cells. PMID: 17475835
- A possible role of Hsp84 in mouse suprachiasmatic nucleus is association with circadian rhythms. PMID: 18480550
- These data provide an explanation for apoptosome inhibition by activated leukemogenic tyrosine kinases and suggest that alterations in Hsp90beta-apoptosome interactions may contribute to chemoresistance in leukemias. PMID: 18591256
- analyzed meiotic maturation in wild-type oocytes treated with a specific inhibitor of Hsp90, 17-allylamino-17-demethoxy-geldanamycin, and observed similar defects PMID: 19158073
- Findings suggest that Hsp90 inhibitors might represent a novel means of promoting T cell tolerance. PMID: 19586661
显示更多
收起更多
-
亚细胞定位:Cytoplasm. Melanosome. Nucleus. Secreted. Cell membrane. Dynein axonemal particle.
-
蛋白家族:Heat shock protein 90 family
-
数据库链接:
KEGG: mmu:15516
STRING: 10090.ENSMUSP00000024739
UniGene: Mm.2180
Most popular with customers
-
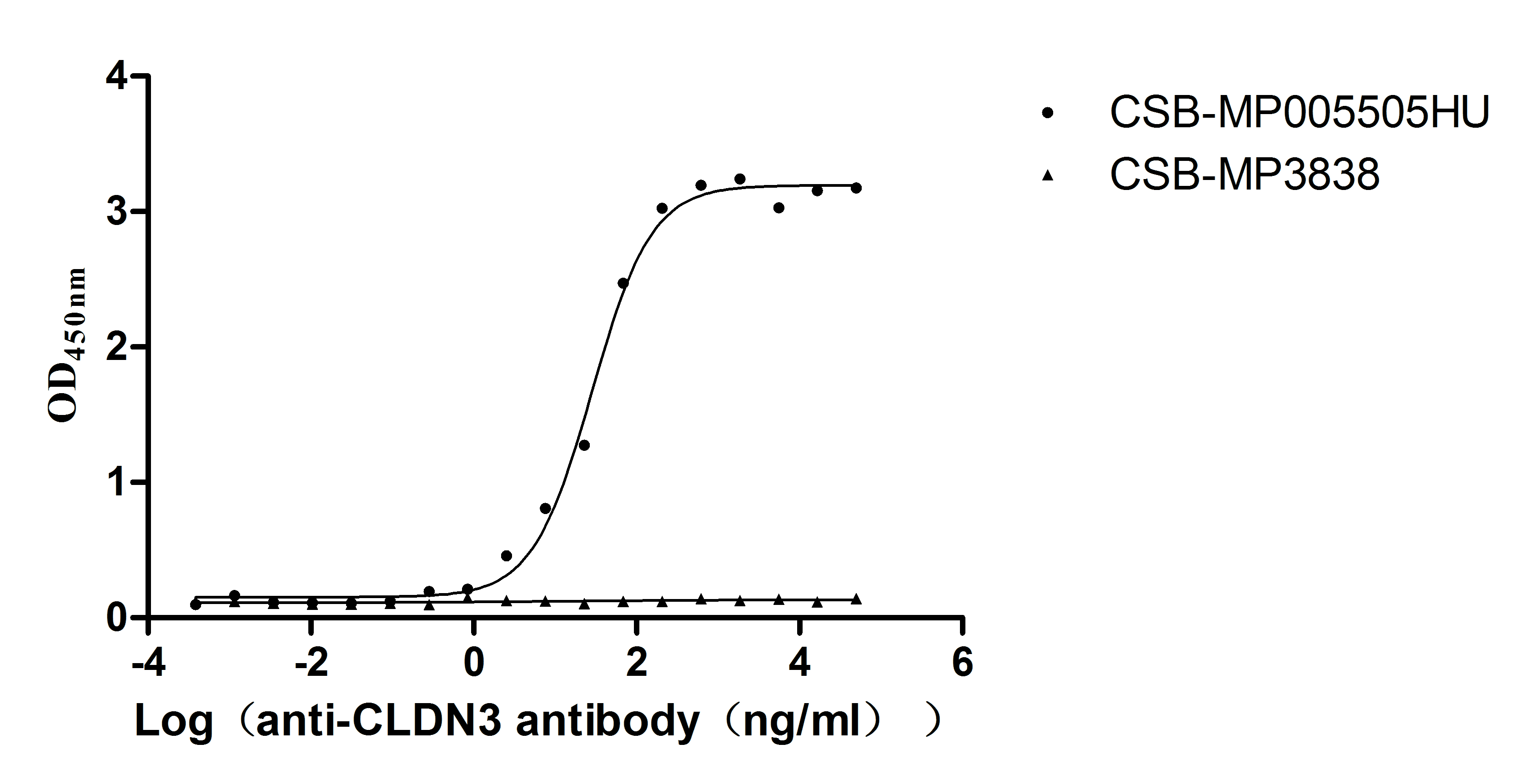
Recombinant Human Claudin-3 (CLDN3)-VLPs (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
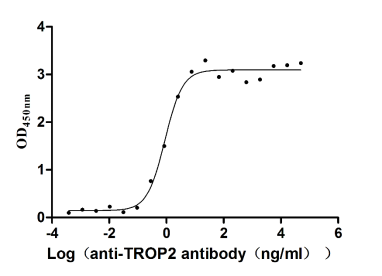
Recombinant Human Tumor-associated calcium signal transducer 2 (TACSTD2), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Cell adhesion molecule 1 (CADM1), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
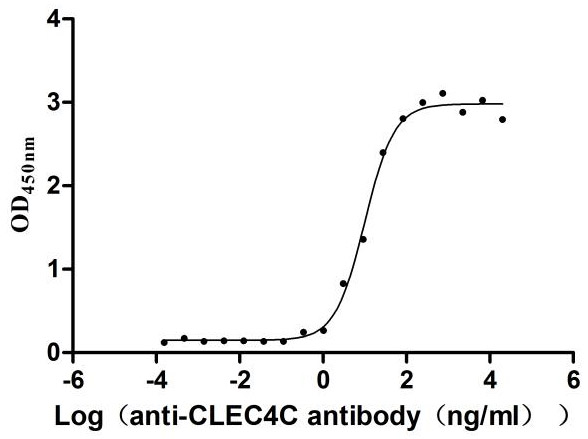
Recombinant Human C-type lectin domain family 4 member C (CLEC4C), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
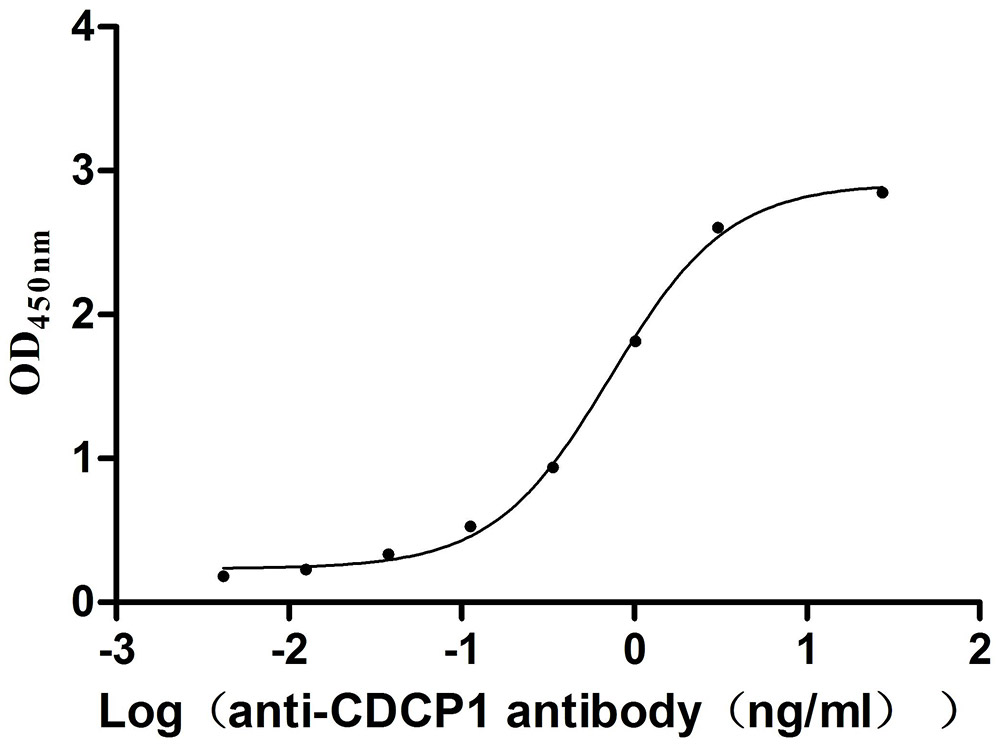
Recombinant Mouse CUB domain-containing protein 1 (Cdcp1), partial (Active)
Express system: Mammalian cell
Species: Mus musculus (Mouse)
-
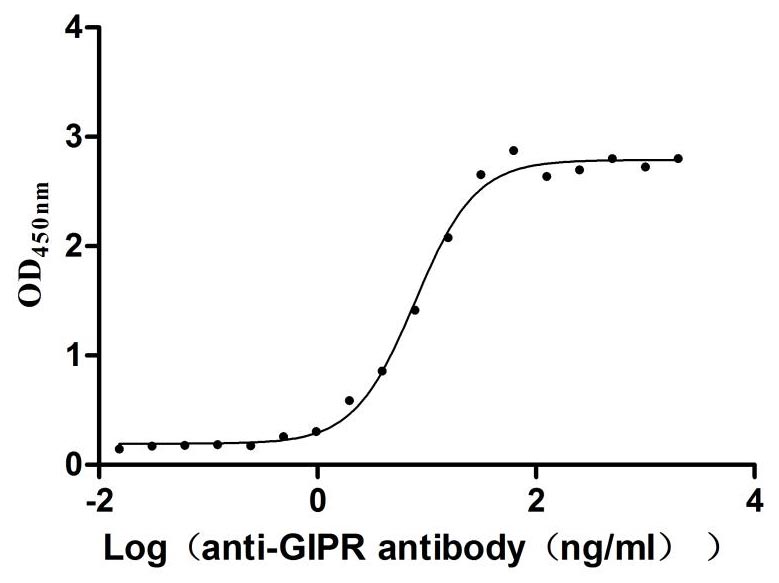
Recombinant Rat Gastric inhibitory polypeptide receptor (Gipr), partial (Active)
Express system: Mammalian cell
Species: Rattus norvegicus (Rat)
-
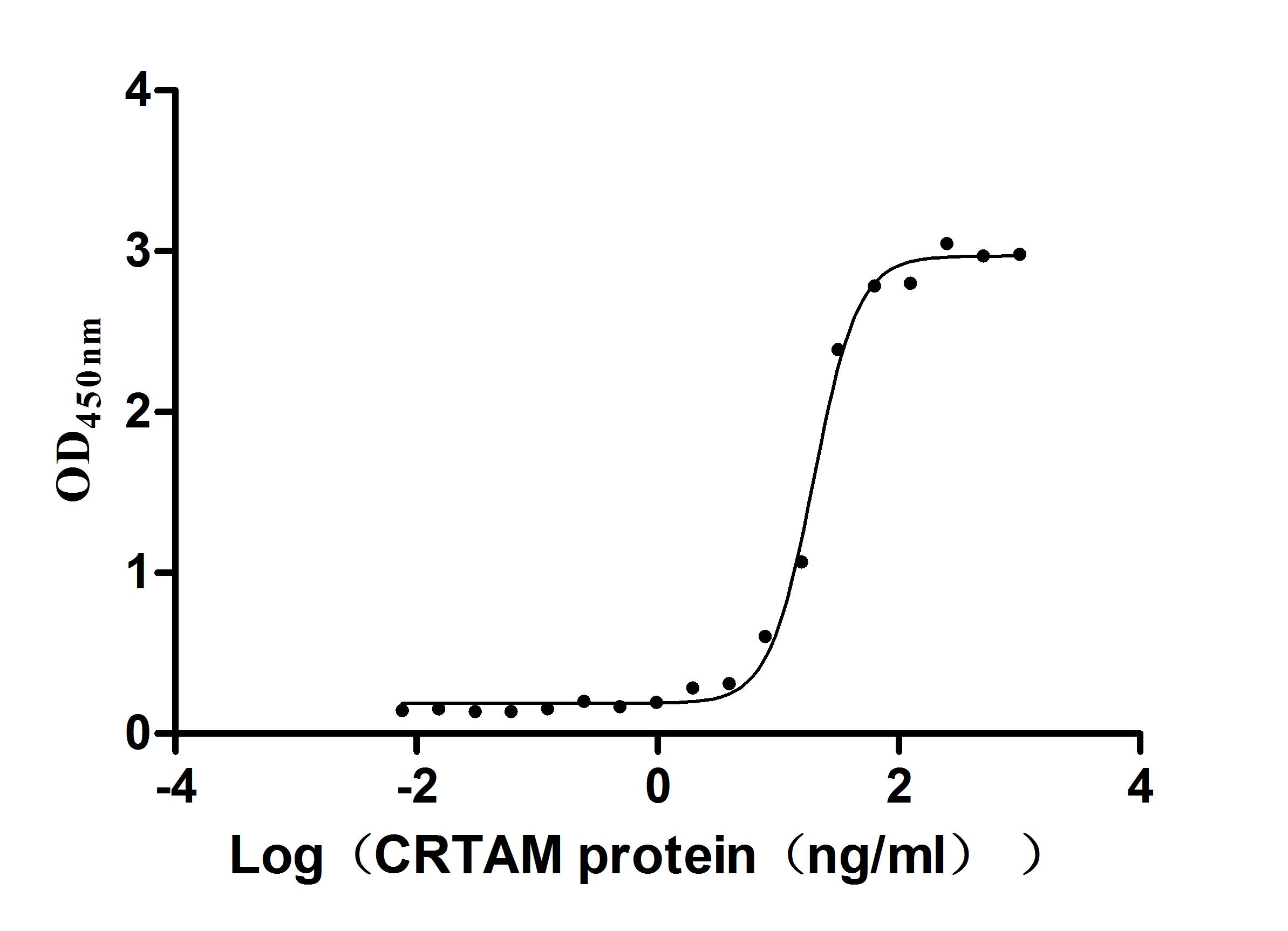
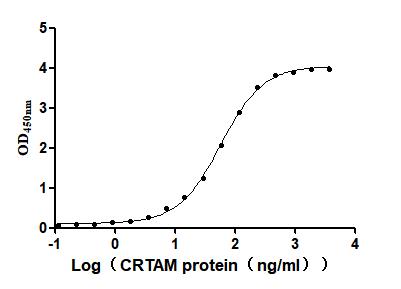
Recombinant Mouse Cytotoxic and regulatory T-cell molecule (Crtam), partial (Active)
Express system: Mammalian cell
Species: Mus musculus (Mouse)
-
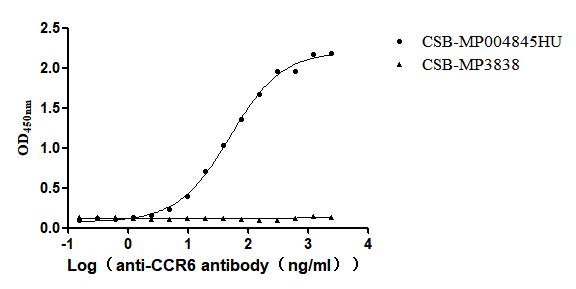
Recombinant Human C-C chemokine receptor type 6(CCR6)-VLPs (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)