Recombinant Mouse Heat shock 70 kDa protein 1A (Hspa1a)
-
货号:CSB-YP717223MO
-
规格:
-
来源:Yeast
-
其他:
-
货号:CSB-EP717223MO
-
规格:
-
来源:E.coli
-
其他:
-
货号:CSB-EP717223MO-B
-
规格:
-
来源:E.coli
-
共轭:Avi-tag Biotinylated
E. coli biotin ligase (BirA) is highly specific in covalently attaching biotin to the 15 amino acid AviTag peptide. This recombinant protein was biotinylated in vivo by AviTag-BirA technology, which method is BriA catalyzes amide linkage between the biotin and the specific lysine of the AviTag.
-
其他:
-
货号:CSB-BP717223MO
-
规格:
-
来源:Baculovirus
-
其他:
-
货号:CSB-MP717223MO
-
规格:
-
来源:Mammalian cell
-
其他:
产品详情
-
纯度:>85% (SDS-PAGE)
-
基因名:Hspa1a
-
Uniprot No.:
-
别名:Hspa1a; Hsp70-3; Hsp70A1Heat shock 70 kDa protein 1A; Heat shock 70 kDa protein 3; HSP70.3; Hsp68
-
种属:Mus musculus (Mouse)
-
蛋白长度:Full Length of Mature Protein
-
表达区域:2-641
-
氨基酸序列AKNTAIGID LGTTYSCVGV FQHGKVEIIA NDQGNRTTPS YVAFTDTERL IGDAAKNQVA LNPQNTVFDA KRLIGRKFGD AVVQSDMKHW PFQVVNDGDK PKVQVNYKGE SRSFFPEEIS SMVLTKMKEI AEAYLGHPVT NAVITVPAYF NDSQRQATKD AGVIAGLNVL RIINEPTAAA IAYGLDRTGK GERNVLIFDL GGGTFDVSIL TIDDGIFEVK ATAGDTHLGG EDFDNRLVSH FVEEFKRKHK KDISQNKRAV RRLRTACERA KRTLSSSTQA SLEIDSLFEG IDFYTSITRA RFEELCSDLF RGTLEPVEKA LRDAKMDKAQ IHDLVLVGGS TRIPKVQKLL QDFFNGRDLN KSINPDEAVA YGAAVQAAIL MGDKSENVQD LLLLDVAPLS LGLETAGGVM TALIKRNSTI PTKQTQTFTT YSDNQPGVLI QVYEGERAMT RDNNLLGRFE LSGIPPAPRG VPQIEVTFDI DANGILNVTA TDKSTGKANK ITITNDKGRL SKEEIERMVQ EAERYKAEDE VQRDRVAAKN ALESYAFNMK SAVEDEGLKG KLSEADKKKV LDKCQEVISW LDSNTLADKE EFVHKREELE RVCSPIISGL YQGAGAPGAG GFGAQAPKGA SGSGPTIEEV D
-
蛋白标签:Tag type will be determined during the manufacturing process.
The tag type will be determined during production process. If you have specified tag type, please tell us and we will develop the specified tag preferentially. -
产品提供形式:Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
复溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
储存条件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保质期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
货期:Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事项:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet :Please contact us to get it.
相关产品
靶点详情
-
功能:Molecular chaperone implicated in a wide variety of cellular processes, including protection of the proteome from stress, folding and transport of newly synthesized polypeptides, activation of proteolysis of misfolded proteins and the formation and dissociation of protein complexes. Plays a pivotal role in the protein quality control system, ensuring the correct folding of proteins, the re-folding of misfolded proteins and controlling the targeting of proteins for subsequent degradation. This is achieved through cycles of ATP binding, ATP hydrolysis and ADP release, mediated by co-chaperones. The co-chaperones have been shown to not only regulate different steps of the ATPase cycle, but they also have an individual specificity such that one co-chaperone may promote folding of a substrate while another may promote degradation. The affinity for polypeptides is regulated by its nucleotide bound state. In the ATP-bound form, it has a low affinity for substrate proteins. However, upon hydrolysis of the ATP to ADP, it undergoes a conformational change that increases its affinity for substrate proteins. It goes through repeated cycles of ATP hydrolysis and nucleotide exchange, which permits cycles of substrate binding and release. The co-chaperones are of three types: J-domain co-chaperones such as HSP40s (stimulate ATPase hydrolysis by HSP70), the nucleotide exchange factors (NEF) such as BAG1/2/3 (facilitate conversion of HSP70 from the ADP-bound to the ATP-bound state thereby promoting substrate release), and the TPR domain chaperones such as HOPX and STUB1. Maintains protein homeostasis during cellular stress through two opposing mechanisms: protein refolding and degradation. Its acetylation/deacetylation state determines whether it functions in protein refolding or protein degradation by controlling the competitive binding of co-chaperones HOPX and STUB1. During the early stress response, the acetylated form binds to HOPX which assists in chaperone-mediated protein refolding, thereafter, it is deacetylated and binds to ubiquitin ligase STUB1 that promotes ubiquitin-mediated protein degradation. Regulates centrosome integrity during mitosis, and is required for the maintenance of a functional mitotic centrosome that supports the assembly of a bipolar mitotic spindle. Enhances STUB1-mediated SMAD3 ubiquitination and degradation and facilitates STUB1-mediated inhibition of TGF-beta signaling. Essential for STUB1-mediated ubiquitination and degradation of FOXP3 in regulatory T-cells (Treg) during inflammation. Negatively regulates heat shock-induced HSF1 transcriptional activity during the attenuation and recovery phase period of the heat shock response.
-
基因功能参考文献:
- CM-695, a small molecule that induces the expression of the HSPA1A/B genes, increases the vessel wall levels of Hsp70 and prevents thrombosis at least as efficiently as rivaroxaban without increasing bleeding risk. PMID: 28837204
- HSPA1A and HSPA8 have roles in parturition through stimulating immune inflammatory and estrogen response PMID: 28025138
- HSPA1A/B induction is a novel approach to delay thrombus formation with minimal bleeding risk in knockout mice. PMID: 26976620
- HspA1A-phosphoinositide binding is complex yet specific, is mediated by both electrostatic and hydrophobic interactions, is not related to the lipid-head charge, and depends on the physicochemical properties of the lipid. PMID: 26923070
- The protective mechanism of HAS2 involves an increased expression of the heat-shock protein Hsp72, a chaperone with antiapoptotic activity PMID: 25555205
- HspA1A embeds in membranes when bound to liposomes composed of cardiolipin and sulfatide.The two domains of HspA1A differentially bind to lipids, such as cardiolipin and sulfatide. PMID: 26476215
- Knockdown lines were created for specific DSBs in regions of the chromosome that are coding for HSPA1A. Clonogenic cell survival was significantly lower in irradiated Hsp70 KD cells with low mHsp70 expression, than in ctrl cells. PMID: 26197988
- genetic manipulation of Hsp72 does not lead to alterations in metabolic processes in cardiac tissue under resting conditions, but identifies mouse strain-specific differences in cardiac lipid accumulation and insulin-stimulated glucose clearance. PMID: 25618331
- Data indicate that heat shock protein 70 (Hsp70) is higher in nuclear protein 1 (Nupr1+/-) haplodeficient mice, concomitant with improved insulin sensitivity. PMID: 25638293
- HuR does not play a role in APA of the Hsp70.3 mRNA, and these two regulatory events appear to be mutually exclusive regulators of Hsp70.3 expression PMID: 25727182
- Activating HSP72 in rodent skeletal muscle increases mitochondrial number and oxidative capacity and decreases insulin resistance. PMID: 24430435
- Activation of HSP-72 upregulates lung injury associated with Pseudomonas aeruginosa pneumonia. PMID: 24667831
- In HSP72 knockout mice, impaired Parkin action was associated with retention of enlarged, dysmorphic mitochondria and paralleled by reduced muscle respiratory capacity, lipid accumulation, and muscle insulin resistance. PMID: 24379352
- Pleural mesothelial cells can release Hsp72 in response to bacterial infection. PMID: 23704948
- In heat stress conditions, Hsp73 is mobilized to prevent apoptosis in the testes and epididymis, and assists Hsp72 in the repair of stress-altered protein conformations. PMID: 23352621
- Hsp72 had greater affinity for tau than Hsc70, but Hsc70 was 30 times more abundant than Hsp72 in human and mouse brain tissue. PMID: 23271055
- Toll-like receptor agonists and febrile range hyperthermia synergize to induce heat shock protein 70 expression and extracellular release PMID: 23212905
- Hsp72 overexpression accelerates the recovery from acute pancreatitis and may represent a potential treatment strategy. PMID: 22792201
- These data indicate that early modulation of astrocyte activation provides an additional novel mechanism associated with Hsp72 overexpression in the setting of ischemia. PMID: 22940431
- alternative polyadenylation of Hsp70.3 in parallel with ischemic or heat shock-induced up-regulation of mRNA levels and implicate the importance of this process in post-transcriptional control of Hsp70.3 expression. PMID: 21757701
- Hsp72 has an essential role in Her2-induced tumorigenesis by regulating oncogene-induced senescence pathways PMID: 21297664
- Upregulation of heat shock protein 72 by geldanamycin reduces brain injury in intracerebral hemorrhage. PMID: 20849898
- Hsp72 reduced infarct area lost and improved behavioral outcome following transient focal cerebralischemia PMID: 21108992
- C-terminus Hsp72 induced tolerance to subsequent LPS stimulation, whereas N-terminus Hsp72 did not induce tolerance PMID: 21094186
- stress-induced GlcNAcylation increases HSF1 expression through inhibition of GSK-3beta, but decreases HSP72 PMID: 20926391
- The results of infarct studies confirm that Hsp70.3 is protective after ischemic preconditioning. PMID: 20643136
- confirmed that radiation therapy induces Hsp72 release primarily from implanted tumors PMID: 20430459
- enhancing astrocyte resistance to ischemic stress by overexpressing either the cytosolic protein Hsp72 or the mitochondrial protein SOD2 resulted in improved survival of CA1 neurons following forebrain ischemia PMID: 20235222
- BAG3 alters the interaction between HSP70 and IKKgamma, increasing availability of IKKgamma and protecting it from proteasome-dependent degradation; this, in turn, results in increased NF-kappaB activity and survival PMID: 20368414
- inducible HSP70.1 and HSP70.3 are required for late-phase protection against myocardial infarction following ischemia preconditioning in mice. PMID: 12714332
- The increased HSP72 expression of S(selection) mice is not a simple proximate effect of their increased wheel running. S mice had significantly elevated HSP72 expression only when housed with free wheels. PMID: 14672969
- Exposure to ionizing radiation led to more residual chromosome aberrations, radioresistant DNA synthesis (a hallmark of genomic instability), increased cell killing, and enhanced IR-induced oncogenic transformation in Hsp70.1/3(-/-) cells. PMID: 14701760
- rapidly induced by Staphylococcus aureus enterotoxin b in intestinal epithelial cells, both directly and through lymphocyte activation (Hsp72) PMID: 15155620
- Hsp72 inhibits apoptosis upstream of the mitochondria and not through interactions with Apaf-1 PMID: 15371421
- helix C is involved in the self-association of Hsp72. PMID: 15498567
- These findings indicate that expression of hsp72 in the hippocampus varies as a function of the learning performance independently from exposure to chronic acoustic stress. PMID: 15719414
- Hsp70 from hsp70.3 may be an initial retinal chaperone while hsp70 from hsp70.1 may be a sustained chaperone. PMID: 15988927
- These results support a multifactoral protective effect of HSP72, some aspects dependent on nuclear localization with stress and some not. PMID: 16100242
- Data show that conditioned media from Lactobacillus GG induce expression of heat shock protein (Hsp)25 and 72 in intestinal epithelial cells. PMID: 16306130
- neuroprotective effects of Hsp70 overexpression in neonatal hypoxic/ischemic injury are mediated by apoptotic pathways through upregulation of FLIP and sequestering Apaf-1, leading to reduced cleavage of caspase-8 and caspase-9 PMID: 16397188
- We report that preconditioning increased HSP72 and heme-oxygenase-1 (HO-1) at 6 and 24 hours of reperfusion, respectively. Unlike nonsteatotic livers, steatotic livers benefited from HSP72 activators (geranylgeranylacetone) throughout reperfusion. PMID: 16651615
- eHsp72 was present in plasma and pulmonary edema (PE)fluid of ALI patients and it was significantly higher in PE fluid from patients with preserved alveolar epithelial fluid clearance. eHsp72 may serve as a marker of SPR activation of ALI patients. PMID: 16679378
- Deletion of Hsp70 genes might induce cardiac dysfunction and development of cardiac hypertrophy through the activation of JNK, p38-MAPK, Raf-1, and ERK. PMID: 16735677
- results indicate that hsp72 levels can serve as a host determinant of viral neurovirulence in C57BL/6 mice, reflecting the direct influence of hsp72 on viral gene expression PMID: 16971451
- These competitive experiments imply that there may be at least two membrane receptors on P388D1 cells and also that both receptors may recognize the various structures in the C-terminal region of the Hsp70 family for regulation of innate immunity. PMID: 17126904
- Heat-stress further enhanced the GGA-related up-regulation of HSP72. PMID: 17482577
- Results suggest an important role for KLF4 as a novel regulator of the constitutive expression of HSP73, but not HSP72. PMID: 18379898
- the absence of interleukin-6 is associated with reduced skeletal muscle Hsp72 PMID: 18927263
- These results suggest that Hspa1a and Hspa1b play an important role in protecting embryos from hyperthermia-induced congenital defects. PMID: 19639652
显示更多
收起更多
-
亚细胞定位:Cytoplasm. Nucleus. Cytoplasm, cytoskeleton, microtubule organizing center, centrosome. Secreted.
-
蛋白家族:Heat shock protein 70 family
-
数据库链接:
KEGG: mmu:193740
STRING: 10090.ENSMUSP00000084586
UniGene: Mm.6388
Most popular with customers
-
Recombinant Human Poliovirus receptor (PVR) (I340M), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
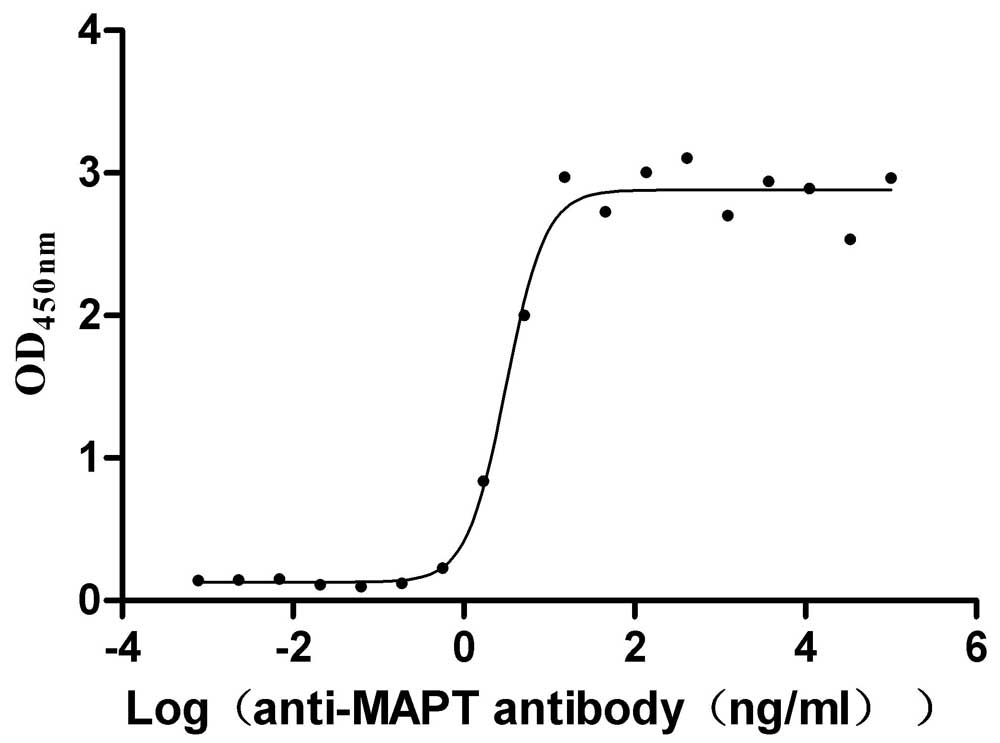
Recombinant Macaca mulatta Microtubule-associated protein tau (MAPT) (Active)
Express system: Mammalian cell
Species: Macaca mulatta (Rhesus macaque)
-
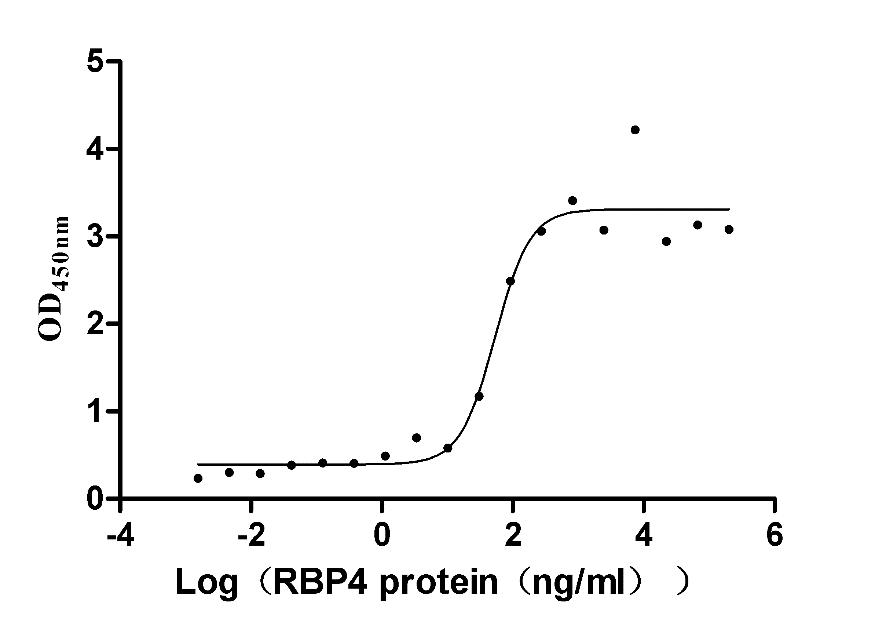
Recombinant Mouse Retinol-binding protein 4 (Rbp4) (Active)
Express system: Mammalian cell
Species: Mus musculus (Mouse)
-
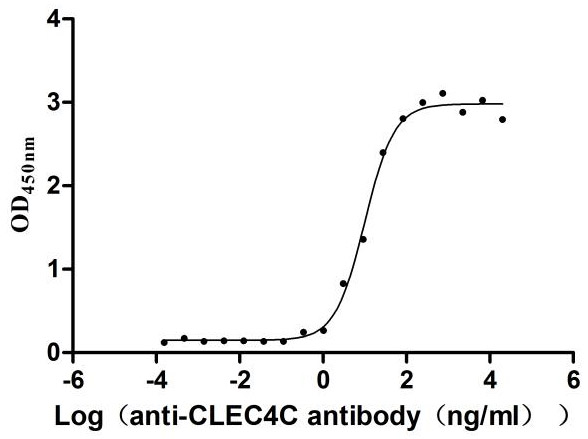
Recombinant Human C-type lectin domain family 4 member C (CLEC4C), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Macaca fascicularis CUB domain containing protein 1 (CDCP1), partial (Active)
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)
-
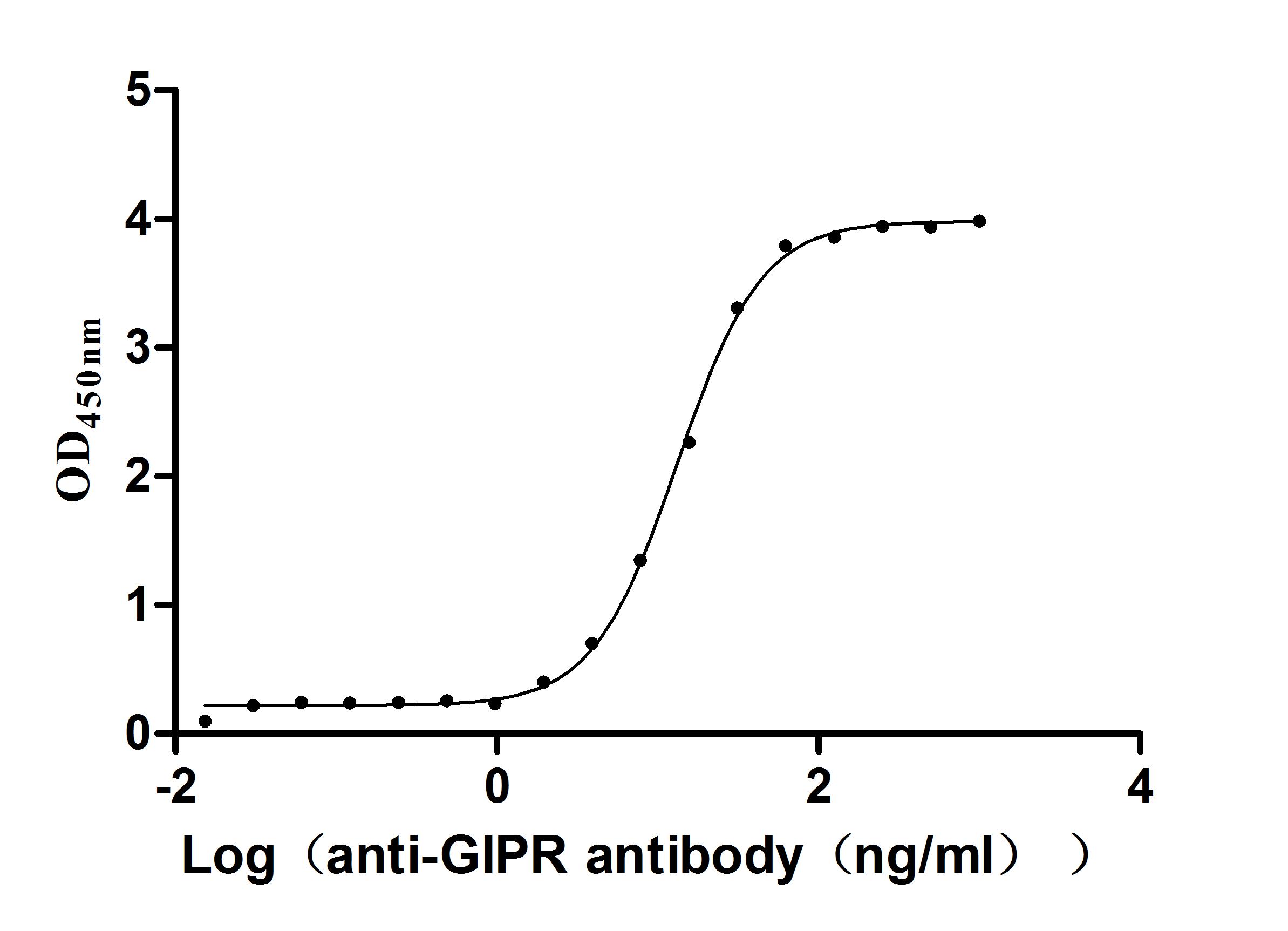
Recombinant Macaca Gastric inhibitory polypeptide receptor(GIPR), partial (Active)
Express system: yeast
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)


-AC1.jpg)