Recombinant Human SRSF protein kinase 1 (SRPK1)
-
中文名称:人SRPK1重组蛋白
-
货号:CSB-YP847224HU
-
规格:
-
来源:Yeast
-
其他:
-
中文名称:人SRPK1重组蛋白
-
货号:CSB-EP847224HU
-
规格:
-
来源:E.coli
-
其他:
-
中文名称:人SRPK1重组蛋白
-
货号:CSB-EP847224HU-B
-
规格:
-
来源:E.coli
-
共轭:Avi-tag Biotinylated
E. coli biotin ligase (BirA) is highly specific in covalently attaching biotin to the 15 amino acid AviTag peptide. This recombinant protein was biotinylated in vivo by AviTag-BirA technology, which method is BriA catalyzes amide linkage between the biotin and the specific lysine of the AviTag.
-
其他:
-
中文名称:人SRPK1重组蛋白
-
货号:CSB-BP847224HU
-
规格:
-
来源:Baculovirus
-
其他:
-
中文名称:人SRPK1重组蛋白
-
货号:CSB-MP847224HU
-
规格:
-
来源:Mammalian cell
-
其他:
产品详情
-
纯度:>85% (SDS-PAGE)
-
基因名:SRPK1
-
Uniprot No.:
-
别名:Serine/arginine rich protein specific kinase 1; Serine/arginine rich splicing factor kinase 1; Serine/arginine-rich protein-specific kinase 1; Serine/threonine protein kinase SRPK1; Serine/threonine-protein kinase SRPK1; SFRS protein kinase 1; SFRSK1; SR protein kinase 1; SR protein specific kinase 1; SR-protein-specific kinase 1; SRPK1; SRPK1_HUMAN
-
种属:Homo sapiens (Human)
-
蛋白长度:full length protein
-
表达区域:1-655
-
氨基酸序列MERKVLALQA RKKRTKAKKD KAQRKSETQH RGSAPHSESD LPEQEEEILG SDDDEQEDPN DYCKGGYHLV KIGDLFNGRY HVIRKLGWGH FSTVWLSWDI QGKKFVAMKV VKSAEHYTET ALDEIRLLKS VRNSDPNDPN REMVVQLLDD FKISGVNGTH ICMVFEVLGH HLLKWIIKSN YQGLPLPCVK KIIQQVLQGL DYLHTKCRII HTDIKPENIL LSVNEQYIRR LAAEATEWQR SGAPPPSGSA VSTAPQPKPA DKMSKNKKKK LKKKQKRQAE LLEKRMQEIE EMEKESGPGQ KRPNKQEESE SPVERPLKEN PPNKMTQEKL EESSTIGQDQ TLMERDTEGG AAEINCNGVI EVINYTQNSN NETLRHKEDL HNANDCDVQN LNQESSFLSS QNGDSSTSQE TDSCTPITSE VSDTMVCQSS STVGQSFSEQ HISQLQESIR AEIPCEDEQE QEHNGPLDNK GKSTAGNFLV NPLEPKNAEK LKVKIADLGN ACWVHKHFTE DIQTRQYRSL EVLIGSGYNT PADIWSTACM AFELATGDYL FEPHSGEEYT RDEDHIALII ELLGKVPRKL IVAGKYSKEF FTKKGDLKHI TKLKPWGLFE VLVEKYEWSQ EEAAGFTDFL LPMLELIPEK RATAAECLRH PWLNS
-
蛋白标签:Tag type will be determined during the manufacturing process.
The tag type will be determined during production process. If you have specified tag type, please tell us and we will develop the specified tag preferentially. -
产品提供形式:Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
复溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
储存条件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保质期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
货期:Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事项:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet :Please contact us to get it.
相关产品
靶点详情
-
功能:Serine/arginine-rich protein-specific kinase which specifically phosphorylates its substrates at serine residues located in regions rich in arginine/serine dipeptides, known as RS domains and is involved in the phosphorylation of SR splicing factors and the regulation of splicing. Plays a central role in the regulatory network for splicing, controlling the intranuclear distribution of splicing factors in interphase cells and the reorganization of nuclear speckles during mitosis. Can influence additional steps of mRNA maturation, as well as other...显示更多
-
基因功能参考文献:
- We now show that the ability of SRPK1 to mobilize SRSF1 from speckles to the nucleoplasm is dependent on active CLK1. Diffusion from speckles is promoted by the formation of an SRPK1-CLK1 complex that facilitates dissociation of SRSF1 from CLK1 and enhances the phosphorylation of several serine-proline dipeptides in this SR protein PMID: 29335301
- SRPK1 is a direct target of miR-1296 in the epatocellular carcinoma. PMID: 28606154
- knockdown of the SRPK1 gene by RNAi inhibits the proliferation of K562 cells and induces apoptosis. PMID: 29138847
- Findings suggest MALAT1 increases AKAP-9 expression by promoting SRPK1-catalyzed SRSF1 phosphorylation in CRC cells. These results reveal a novel molecular mechanism by which MALAT1 regulates AKAP-9 expression in CRC cells. PMID: 26887056
- The frameshift mutation detected in the current study would result in premature stops of amino acid synthesis in PLA2R1 and SRPK1 proteins and hence resembles a typical inactivating mutation. PMID: 28212809
- Data suggest that ERH interacts directly in nucleus with C-terminal Arg-Gly-rich region of SAFB1/SAFB2 and this multimer co-localizes in insoluble nuclear fraction; binding of ERH reverses inhibition exerted by SAFB1/SAFB2 on SRPK1. (ERH = enhancer of rudimentary homolog protein; SAFB = scaffold attachment factor B; SRPK1 = splicing kinase SR protein kinase-1) PMID: 28627136
- SRPK1 interacts with an RS-like domain in the N terminus of CLK1 to facilitate the release of phosphorylated SR proteins, which then promotes efficient splice-site recognition and subsequent spliceosome assembly. PMID: 27397683
- Data indicate that proto-oncogene protein c-akt (Akt) phosphorylates distinct sites than SRPK1 protein within the arginine-serine (RS) domain of Lamin B Receptor (LBR). PMID: 27105349
- SRPK1 interference inhibits the growth and invasion of RCC cells. PMID: 27662590
- SRPK1 is overexpressed in HCC and may be a promising indicator of prognosis for HCC patients PMID: 26201897
- Overexpression of SRPK1 is associated with glioma. PMID: 26738865
- The mRNA level and the protein level of SRPK1 were up-regulated in NSCLC tissues. It activated the transcriptional activity of beta-catenin/T-cell factor complex. SRPK1 knockdown attenuated the expression of target genes of this complex. Silencing SRPK1 down-regulated the phosphorylation of GSK3beta. PMID: 26666824
- SRPK1 is upregulated in prostate cancer and correlates with disease stage and invasion. PMID: 26500332
- Data indicate serine/arginine-rich protein kinases SRPK1/2/SRPIN340 complexes by molecular docking and molecular dynamics. PMID: 26244849
- SRPK1 mediates TGF-beta-induced proliferation. PMID: 26099172
- SRPK1 may be a potential anticancer target to inhibit glioma progression. PMID: 25833691
- Modulation of SRPK1 and subsequent inhibition of tumour angiogenesis. PMID: 25381816
- SRPK1 is a regulator of Tra2beta1 splicing function and individual domains engage in considerable cross-talk, assuming novel functions with regard to RNA binding, splicing, and catalysis. PMID: 26013829
- SRPK1 was highly expressed in basal breast cancer. High SRPK1 expression correlated with poor breast cancer disease outcome and preferential metastasis to the lungs and brain. PMID: 25774502
- Increased expression of SRPK1 correlates with the progression of breast cancer.SRPK1 is upregulated in breast cancer at both mRNA and protein levels. PMID: 24961466
- Data suggest that proline phosphorylation by CLK1/CDC-like kinase 1 (but not by SRPK1/serine/arginine-rich splicing factor kinase 1) regulates conformation and alternative splicing function of SFRS1 (serine/arginine-rich splicing factor 1). PMID: 25529026
- Collectively, these data suggest that human papillomavirus type 1 E1;E4-mediated inhibition of SRPK1 could affect the functions of host serine-arginine proteins and those of the virus transcription/replication regulator E2. PMID: 25142587
- s show that MNK regulates SRPK via mTOR and AKT. PMID: 25187540
- ERK1/2 signal induced MNK catalytic activity enabled enterovirus type 1 internal ribosomal entry site-mediated translation/host cell cytotoxicity through negative regulation of the Ser/Arg (SR)-rich protein kinase (SRPK). PMID: 25187541
- Hypoxic stress decreased the miR-9 level in ARPE-19 cells, which increased the transcriptional level of SRPK-1, resulting in alternative splicing shift to pro-angiogenic isoforms of VEGF165 in human retinal pigment epithelial cells. PMID: 25007957
- Up-regulation of SRPK1 is associated with breast cancer. PMID: 25140042
- Inhibition of SRPK1 by knockdown or with pharmacological inhibitors reduces expression of pro-angiogenic forms of VEGF. PMID: 25010863
- The data show that the RS domain in SRSF1 is multifunctional and that sequences once thought to be catalytically silent can be recruited to enhance the efficiency of SR protein phosphorylation. PMID: 24984036
- positively regulates IFN-lambda1 genes during viral infection PMID: 23405030
- Data suggest that the conformation of SRPK1 is highly flexible and can readily adapt to changes in structure of arginine-serine rich domains in substrate proteins such as SRSF1 (serine-arginine-rich splicing protein 1). PMID: 24074032
- SRPK1 plays an oncogenic role in hepatocellular carcinoma through a possible mechanism involving PI3K/Akt. PMID: 23644876
- The data establish a new view of SRSF1 protein regulation in which SRPK1 and CLK1 partition activities based on Ser-Pro versus Arg-Ser placement rather than on N- and C-terminal preferences along the RS domain. PMID: 23707382
- These findings reveal a major signal transduction pathway for regulated splicing and place SRPKs in a central position in the pathway, consistent with their reputed roles in a large number of human cancers. PMID: 22727668
- The present immunohistochemical study reveals a region- and neuron-specific localization of SRPK1 in human brain. PMID: 22019390
- WT1 bound to the SRPK1 promoter, and repressed expression through a specific WT1 binding site PMID: 22172722
- protein kinase SRPK1 phosphorylates ~10 serines in the arginine--serine-rich domain (RS domain) of the SR protein SRSF1 in a C- to N-terminal direction, a modification that directs this essential splicing factor from the cytoplasm to the nucleus PMID: 21728354
- SRPK1a may play an important role in linking ribosomal assembly and/or function to erythroid differentiation in human leukaemic cells PMID: 20708644
- Our results demonstrate that SRPK1 and SRPK2 are host factors essential for Hepatitis C virus replication. PMID: 20498328
- Both SRPK1 and SRPK2 are most likely the cellular protein kinases mediating HBV core protein phosphorylation during viral infection and therefore represent important host cell targets for therapeutic intervention in HBV infection. PMID: 12134018
- negative role of SRPK1 and SRPK2 in the regulation of HBV replication through a mechanism not involving the phosphorylation of the core protein PMID: 16122776
- ASF/SF2 is phosphorylated by SRPK1 and Clk/Sty PMID: 16223727
- We describe crystallographic, molecular dynamics, and biochemical results that shed light on how SRPK1 preserves its constitutive active conformation. PMID: 17223538
- Serine-arginine protein kinase 1 overexpression is associated with tumorigenic imbalance in mitogen-activated protein kinase pathways. PMID: 17332336
- SRPK1 binding is associated with phosphorylation of human papillomavirus type 1 E1/E4 polypeptide and modulates autophosphorylation of the kinase. PMID: 17360743
- Sky1p utilizes the same docking groove to bind yeast SR-like protein Gbp2p and phosphorylates all three serines present in a contiguous RS dipeptide stretch PMID: 17517895
- These studies reveal that SRPK1 docks near the C-terminus of the RS1 segment of ASF protein and then moves in an N-terminal direction along the RS domain. PMID: 18155240
- Data show that a sliding docking interaction is essential for sequential and processive phosphorylation of an SR protein by SRPK1. PMID: 18342604
- Data show that adaptable molecular interactions guide phosphorylation of the SR protein ASF/SF2 by SRPK1. PMID: 18687337
- SRPK1, a ubiquitously expressed SR protein-specific kinase, directly binds to the cochaperones Hsp40/DNAjc8 and Aha1, which mediate dynamic interactions of the kinase with the major molecular chaperones Hsp70 and Hsp90 in mammalian cells PMID: 19240134
- while local RS/SR content steers regional preferences in the RS domain, distal contacts with SRPK1 guide initiation and directional phosphorylation within these regions. PMID: 19477182
收起更多
-
亚细胞定位:[Isoform 2]: Cytoplasm. Nucleus. Nucleus matrix. Microsome.
-
蛋白家族:Protein kinase superfamily, CMGC Ser/Thr protein kinase family
-
组织特异性:Isoform 2 is predominantly expressed in the testis but is also present at lower levels in heart, ovary, small intestine, liver, kidney, pancreas and skeletal muscle. Isoform 1 is only seen in the testis, at lower levels than isoform 2. Highly expressed in
-
数据库链接:
HGNC: 11305
OMIM: 601939
KEGG: hsa:6732
STRING: 9606.ENSP00000362931
UniGene: Hs.443861


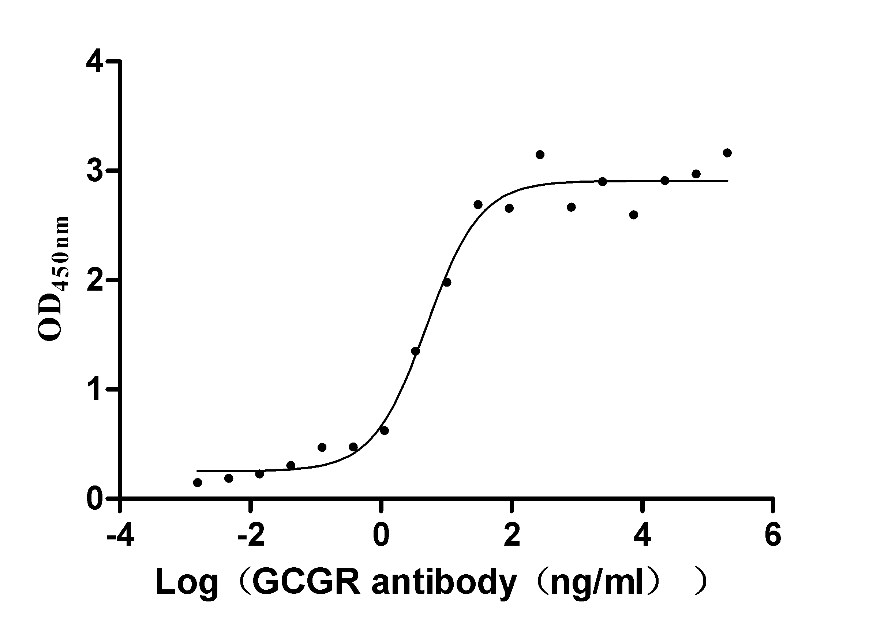
-AC1.jpg)