Recombinant Human Islet amyloid polypeptide protein (IAPP), partial
In Stock-
货号:CSB-EP010931HU
-
规格:¥1344
-
图片:
-
其他:
产品详情
-
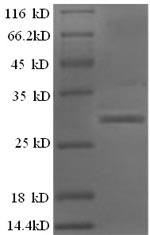
纯度:Greater than 90% as determined by SDS-PAGE.
-
基因名:
-
Uniprot No.:
-
别名:Amylin; DAP; Diabetes associated peptide; Diabetes-associated peptide; IAP; IAPP; IAPP_HUMAN; Insulinoma amyloid peptide; Islet amyloid polypeptide (diabetes associated peptide, amylin); Islet amyloid polypeptide
-
种属:Homo sapiens (Human)
-
蛋白长度:partial
-
来源:E.coli
-
分子量:31.4kDa
-
表达区域:34-70aa
-
氨基酸序列KCNTATCATQRLANFLVHSSNNFGAILSSTNVGSNTY
Note: The complete sequence including tag sequence, target protein sequence and linker sequence could be provided upon request. -
蛋白标签:N-terminal GST-tagged
-
产品提供形式:Liquid or Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
缓冲液:Tris-based buffer,50% glycerol
-
储存条件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保质期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
货期:3-7 business days
-
注意事项:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet & COA:Please contact us to get it.
相关产品
靶点详情
-
功能:Selectively inhibits insulin-stimulated glucose utilization and glycogen deposition in muscle, while not affecting adipocyte glucose metabolism.
-
基因功能参考文献:
- In conclusion, IAPP/amylin directly interacts with NLRP3 to activate NLRP3 inflammasome, and this interaction could be an attractive drug target to avoid inflammation and beta-cell death during therapy for diabetes, although there are mechanisms in NLRP3 inflammasome and diabetes pathology in human tissues that still require elucidation. PMID: 30014749
- These findings suggest that increased membrane permeation induced by oligomeratization of amylin peptide in cell sarcolemma contributes to Ca(2+) dysregulation in pre-diabetes. PMID: 29604965
- down-regulation of IAPP expression induces the death of human annulus fibrosus cells via mitochondrial and death receptor pathways, potentially offering a novel therapeutic target for the treatment of intervertebral disc degeneration. PMID: 28433710
- This study highlights that with positional scanning of the split-tetracysteine motif (Cys-Cys), the fluorogenic probe fluorescein arsenical hairpin detection method offers unique time-dependent conformational insights on the proteospecies assembled throughout the amyloidogenic pathway of IAPP. PMID: 29360346
- Bri2 BRICHOS domain is a potent inhibitor of IAPP fibril formation.IAPP colocalizes with Bri2 both intracellularly and in islet amyloid deposits. PMID: 29507232
- Insulin resistance in rheumatoid arthritis does not appear to be mediated by amylin. This would imply that the mechanisms associated with IR in RA patients differ from those at work in type 2 diabetes. PMID: 29352842
- During aggregation, the nucleating NFGAIL region remains flexible and accessible within this isolated intermediate, suggesting a mechanism by which membrane-associated aggregation may be propagated. PMID: 29148426
- Study presents a new Zn(2+) binding site in the N-terminus of fibrillary amylin with three different coordination modes. Simulations showed that Zn(2+) ions bind to polymorphic amylin fibrils with a preference to bind to four Cys residues rather than two Cys residues of two neighboring amylin monomers. PMID: 28692245
- The IAPP beta-hairpin can serve as a molecular recognition motif enabling control of IAPP aggregation. PMID: 27641459
- cholesterol significantly modulates the ability of model membranes to induce IAPP amyloid formation and IAPP-mediated membrane damage. PMID: 29373018
- point mutations within the central aggregation-prone regions contribute to the reduction of the overall amyloidogenic potential of IAPP but do not completely abolish the formation of IAPP amyloid fibrils. PMID: 28602716
- Using the Tg2576 AD mouse model, a single intraperitoneal injection of amylin significantly increased Abeta serum levels, and the effect was abolished by AC253, an amylin receptor antagonist, suggesting that amylin effect could be mediated by its receptor. Subsequent mechanistic studies showed amylin enhanced Abeta transport across a cell-based model of the blood-brain barrier. PMID: 28059785
- These results suggest that protein segment structures represent polymorphs of their parent protein and that segment 19-29 S20G may serve as a model for the toxic spine of human IAPP. PMID: 28045370
- All-atom explicit-water molecular dynamics (MD) simulations studying adsorption, orientation, and surface interaction of hIAPP aggregates with different sizes (monomer to tetramer) and conformations (monomer with alpha-helix and tetramer with beta-sheet-rich U-turn) upon adsorption. hIAPP monomer with alpha-helical conformation and hIAPP pentamer with beta-sheet conformation can adsorb on both POPC and POPC/POPE bilayers. PMID: 28585804
- The aggregation of rhIAPP also occurred significantly faster when compared with that of the chemically synthesized peptide. PMID: 29046394
- Data (including data from studies using tissues from transgenic mice) suggest that IL1B plays dual roles by (1) mediating islet amyloid-induced FAS up-regulation and apoptosis in pancreatic beta-cells and (2) down-regulating IAPP precursor processing thereby potentiating islet amyloid formation. (IL1B = interleukin-1beta; FAS = FAS cell surface death receptor; IAPP = islet amyloid polypeptide) PMID: 28058779
- Data suggest that single aromatic/hydrophobic amino acid residues within IAPP (islet amyloid polypeptide) amyloid core region are able to control its interaction with amyloid-beta(1-40) or amyloid-beta(1-42) but not IAPP self-assembly; four aromatic/hydrophobic residues are able to control both IAPP amyloid self-assembly and its cross-interaction with amyloid-beta(1-40) or amyloid-beta(1-42). PMID: 28684415
- Data show that aluminum (Al3+) could inhibit islet amyloid polypeptide hIAPP(11-28) fibrillogenesis. PMID: 28338927
- The absence of BACE2 ameliorates glucose tolerance defects induced by IAPP overexpression in the beta-cell and promotes beta-cell survival. PMID: 28337562
- This study supports the elucidation of the structural basis of IAPP amyloid formation and highlights the extent of amyloid fibril polymorphism. PMID: 27607147
- Data suggest that a single GlcNAc residue at CTR N130 (asparagine 130) is responsible for enhanced affinity of calcitonin for CTR ECD; the same appears to apply for enhanced affinity of amylin for RAMP2-CTR ECD. [GlcNAc = N-acetylglucosamine; CTR = calcitonin receptor; ECD = extracellular domain; RAMP2 = receptor (calcitonin) activity modifying protein 2]. PMID: 28614667
- The kinetics of human amylin amyloid formation can be monitored by SYPRO-orange fluorescence and match the time course determined with thioflavin-T assays. PMID: 27479186
- effect of cholesterol on the amyloidogenicity of IAPP PMID: 27410742
- Together, our preclinical results suggest that AT406 could be further evaluated as a promising anti-pancreatic cancer agent. PMID: 27387230
- Al(III) could promote fibrillation and aggregation of hIAPP, while EGCG could inhibit the fibrillation of hIAPP and lead to the formation of hIAPP amorphous aggregates instead of the ordered fibrils PMID: 28074190
- Chondroitin sulfate A has an intensive promotion effect on the fibrillation of human IAPP at the palmitoyloleoylphosphatidylcholine (POPC) membrane, which is larger than the total effect of Chondroitin sulfate A alone and POPC alone. PMID: 27067251
- C4BP protects beta-cells from IAPP cytotoxicity by modulating IAPP fibril formation extracellularly and also, after uptake by the cells, by enhancing cholesterol synthesis. PMID: 27566545
- beta-hairpin peptide inhibitor of IAPP aggregation that are stabilized in that conformation, or even forced to remain in the hairpin conformation by a backbone cyclization constraint, display superior activity as inhibitors. PMID: 27317951
- hA17-29 aggregate toxicity seems to be mediated by RAGE and p75-NGFR receptors PMID: 27804051
- Serum levels of preptin and amylin were significantly lower in patients with psoriasis and Behcet's disease. PMID: 25545917
- These data suggest participation by both soluble and fibrillar aggregates in IAPP-induced islet inflammation. IAPP-induced activation of TLR2 and secretion of IL-1 may be important therapeutic targets to prevent amyloid-associated beta cell dysfunction. PMID: 26786104
- the effect of the endoplasmic reticulum chaperone protein disulfide isomerase (PDI) on beta-cell dysfunction, was examined. PMID: 26607804
- Data suggest that IAPP/membrane interaction strongly depends on protonation state of His18; at neutral pH, N-terminal domain is stabilized/anchored to membrane, while C-terminal domain is disordered as if in solution. PMID: 26953503
- Surface molecular structure and amino acid residue composition of hIAPP fibrils are specifically probed with nanoscale resolution using tip-enhanced Raman spectroscopy. PMID: 25952953
- Structural studies and cytotoxicity assays of "aggregation-prone" IAPP(8-16) and its non-amyloidogenic variants suggest its important role in fibrillogenesis and cytotoxicity of human amylin. PMID: 25913357
- structural and dynamical information of amylin and CGRP PMID: 26331261
- The computational models support the cross-sequence interactions between ABETA and IAPP pentamers, which would lead to the complex hybrid ABETA-IAPP assemblies. PMID: 26173078
- Study focused on human islet amyloid polypeptide aggregates and a de novo designed short polypeptide at lipid/water and air/glass interfaces; found that parallel beta-sheets adopt distinct orientations at various interfaces and exhibit characteristic chiroptical responses in the amide I and N-H stretch regions. PMID: 26263128
- Secondary Structure of Rat and Human Amylin PMID: 26221949
- The intramolecular hydrogen bond formation by His(18) and the possibility of His(18) participating in the formation of alpha-helical structures greatly modulated the manner of hIAPP amyloid formation. PMID: 26777153
- Matrix Metalloproteinase-9 Protects Islets from Amyloid-induced Toxicity. PMID: 26483547
- cross-seeding assemblies between hIAPP and rIAPP oligomers PMID: 25706385
- combined computational and experimental study offers detailed mechanistic insight into the complex role of zinc on IAPP aggregation and T2D development PMID: 26603575
- When copper(II) was present in the solution, no dimers were detected. Thus, the copper(II) ions disrupt the association pathway to the formation of beta-sheet rich amylin fibrils. PMID: 26352401
- Computational studies identified new IAPP mutations that have stronger amyloid fibrils destabilizing potential that the currently known. PMID: 25903685
- The effect of a single proline mutant at position 26 in an amylin polypeptide on its binding to a lipid bilayer was studied. PMID: 25427619
- Inhibition of IAPP aggregation by insulin depends on the insulin oligomeric state regulated by zinc ion concentration PMID: 25649462
- Introducing the porcine insulin promoter-hIAPP expression vector into PK15 cells combined with exogenous Pdx-1, MafA and NeuroD1 resulted in the efficient expression of hIAPP at the gene level, but not the protein. PMID: 24825179
- There was abundant amylin aggregates in lysates of cardiac myocytes from obese patients, but not in controls. amylin aggregation at the sarcolemma induces oxidative stress and Ca(2+) dysregulation. PMID: 25146704
- interaction between HS and IAPP or the subsequent effects represent a possible therapeutic target whose blockage can lead to a prolonged survival of beta cells PMID: 25922077
显示更多
收起更多
-
亚细胞定位:Secreted.
-
蛋白家族:Calcitonin family
-
数据库链接:
HGNC: 5329
OMIM: 147940
KEGG: hsa:3375
STRING: 9606.ENSP00000240652
UniGene: Hs.46835
Most popular with customers
-
Recombinant Human Glucagon receptor (GCGR), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Macaca fascicularis Membrane spanning 4-domains A1 (MS4A1)-VLPs (Active)
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)
-
Recombinant Macaca fascicularis Transmembrane 4 L6 family member 1 (TM4SF1)-VLPs (Active)
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)
-
Recombinant Human Gastric inhibitory polypeptide receptor(GIPR),partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)