Recombinant Human Gamma-crystallin D (CRYGD)
-
货号:CSB-YP006020HU
-
规格:
-
来源:Yeast
-
其他:
-
货号:CSB-EP006020HU-B
-
规格:
-
来源:E.coli
-
共轭:Avi-tag Biotinylated
E. coli biotin ligase (BirA) is highly specific in covalently attaching biotin to the 15 amino acid AviTag peptide. This recombinant protein was biotinylated in vivo by AviTag-BirA technology, which method is BriA catalyzes amide linkage between the biotin and the specific lysine of the AviTag.
-
其他:
-
货号:CSB-BP006020HU
-
规格:
-
来源:Baculovirus
-
其他:
-
货号:CSB-MP006020HU
-
规格:
-
来源:Mammalian cell
-
其他:
产品详情
-
纯度:>85% (SDS-PAGE)
-
基因名:CRYGD
-
Uniprot No.:
-
别名:CACA; CCA3; CCP; CRGD_HUMAN; CRYG4; Crygd; Crystallin; gamma D; Crystallin; gamma-4; CTRCT4; Gamma crystallin D; Gamma D crystallin; Gamma-crystallin 4; Gamma-crystallin D; Gamma-D-crystallin; PCC
-
种属:Homo sapiens (Human)
-
蛋白长度:Full Length of Mature Protein
-
表达区域:2-174
-
氨基酸序列GKITLYEDR GFQGRHYECS SDHPNLQPYL SRCNSARVDS GCWMLYEQPN YSGLQYFLRR GDYADHQQWM GLSDSVRSCR LIPHSGSHRI RLYEREDYRG QMIEFTEDCS CLQDRFRFNE IHSLNVLEGS WVLYELSNYR GRQYLLMPGD YRRYQDWGAT NARVGSLRRV IDFS
-
蛋白标签:Tag type will be determined during the manufacturing process.
The tag type will be determined during production process. If you have specified tag type, please tell us and we will develop the specified tag preferentially. -
产品提供形式:Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
复溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
储存条件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保质期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
货期:Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事项:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet :Please contact us to get it.
相关产品
靶点详情
-
功能:Crystallins are the dominant structural components of the vertebrate eye lens.
-
基因功能参考文献:
- At physiological pH, CRYGD forms aggregates that look amorphous and disordered by electron microscopy. Surprisingly, solid-state NMR reveals that these amorphous deposits have a high degree of structural homogeneity at the atomic level and that the aggregated protein retains a native-like conformation, with no evidence for large-scale misfolding. PMID: 28474685
- A molecular dynamics approach to explore the structural characterization of cataract causing mutation R58H on human gammaD crystallin. PMID: 29532225
- This research compared the effects of various glycation modifiers on Hgammad-crystallin aggregation, by treating samples of Hgammad-crystallin with ribose, galactose, or methylglyoxal using several biophysical techniques PMID: 29949747
- Study reports the identification of Cys111 as the major residue responsible for disulfide formation in protein dimers as well as for Cu2+-induced aggregation of human gammaD-crystallin. PMID: 30251679
- Using the P23T mutant of gammaD-crystallin, a protein associated with congenital cataract, we have demonstrated that the equilibrium solubility boundary and solution behavior measured using phase diagrams of purified protein solutions is consistent with the assembly of the protein expressed in cell-free expression medium in artificial cells (without fluorescent labelling) and condensates formed in mammalian cells. PMID: 28401204
- we identified two heterozygous rare variants in genes that are involved in early cataract development; the novel c.809C>A; p.(Ser270Tyr) in MAF and the c.168C>G; p.(Tyr56 *) variant in CRYGD, previously reported as pathogenic PMID: 28849415
- Aggregation of Trp > Glu point mutants of human gamma-D crystallin provides a model for hereditary or UV-induced cataract. PMID: 26991007
- the mechanism of aggregation of two gammaD-crystallin mutants, W42R and W42Q: the former a congenital cataract mutation PMID: 27417136
- Mutational analysis of CRYGD identified a recurrent (p.P24T) mutation in two unrelated families with congenital coralliform cataracts and three novel (p.Q101X, p.E104fsX4 and p.E135X) mutations in three families with congenital nuclear cataracts. PMID: 26732753
- The nonsense mutation c.471G>A of the CRYGD gene probably underlies the congenital cataract in the pedigree PMID: 27455011
- Single-molecule Force Spectroscopy Predicts a Misfolded, Domain-swapped Conformation in human gammaD-Crystallin Protein. PMID: 26703476
- We have identified a novel mutation, c.451_452insGACT, in CRYGD, which is associated with nuclear cataract. This is the first insertion mutation of CRYGD found to cause autosomal dominant congenital cataract. PMID: 26147294
- We have used trio-based exome sequencing to uncover a recurrent missense mutation in CRYGD and two novel missense mutations in GJA8 associated with autosomal dominant cataract in three nuclear families. PMID: 25403472
- Created are three double mutants of human gamma D-crystallin for which the phase diagrams for singly mutated proteins can be used to predict the behavior of the double mutants. PMID: 25613833
- oxidation-mimicking W42Q mutant of gammad-crystallin formed non-native polymers starting from a native-like state under physiological conditions PMID: 25787081
- Shared epitopes and smoking were associated with the production of anti-CCP antibodies and rheumatoid factors of IgM and IgA isotypes, which again were associated with erosive disease at presentation only in smokers. PMID: 25205362
- The presence of anti-CCP antibodies was a reliable serologic marker in rheumatoid arthritis diagnosis and was associated with cigarette smoking. PMID: 24366391
- These results indicated that the single lysine residue at the second position (K2) is acetylated at an early age and that the amount of K2-acetylated gamma D-crystallin increased with age. PMID: 25393041
- Results show that thermal denaturation of gammaD-crystallin results in sheet-like aggregates that contain cross-linked oligomers of the protein. PMID: 24415662
- This study identified a novel congenital nuclear and posterior polar cataract phenotype caused by the recurrent mutation p. R140X in CRYGD. PMID: 24465161
- Presentation and discussion of the first crystal structure of the P23T mutant at 2.5 A resolution. PMID: 23670788
- Outcome from protein formulation characterization supports the hypothesis that the gammaD-crystallin it is able to recover and improve the mechanical properties of chemical damaged hair. PMID: 23651449
- study establishes that UV-B irradiation of gammaD-Crystallin leads to structurally specific modifications and precipitation via two mechanisms: amorphous aggregates and amyloid fibers PMID: 23957864
- The missense P24T mutation in CRYGD was responsible for the coralliform cataract afflicting a four-generation Chinese family. PMID: 24103489
- Mass spectrometry identifies residues 80-163 as the amyloid core, which spans most of the C-terminal domain, the linker, and a small portion of the N-terminal domain. PMID: 23082813
- human W42R gammaD-crystallin mutant structure provides a link between congenital and age-related cataracts PMID: 23124202
- A missense mutation in CRYGD linked with autosomal dominant congenital cataract of aculeiform type. PMID: 22669729
- study of effects of G61C mutation on gammaD-crystallin structure, stability and aggregation; results suggest the decrease in protein stability followed by aggregation-prone property may be the major cause in hereditary cataract induced by the G61C mutation PMID: 21655238
- The mutations in CRYGD were shown to cause changes in protein surface polarity, hydrophobicity, and spatial structure, contributing to protein deposition and cataract formation. PMID: 18041179
- neither structural nor stability changes in the protein are responsible for the R76S gammaD-crystallin variant's association with cataract PMID: 22394327
- Upon acid-induced amyloid fibril formation, the C-terminal domain forms amyloid beta-sheets, the N-terminal domain becomes extremely disordered but lies near to the beta-sheets. Fibril nucleation & extension occur only in the C-terminal domain. PMID: 22328156
- The heterozygous 109C to A CRYGD missense mutation is associated with a distinct crystalline cataract in two US Caucasian pedigrees. PMID: 22219628
- mutants and possibly age-damaged gammaD-crystallin can escape quality control by lens chaperones rationalizing the observation that they nucleate protein aggregation and lead to cataract PMID: 22289178
- A novel substitution has been found in CRYGD in a Brazilian family with congenital cataract in which there is no segregation with the disease, indicating that it is probably not a disease-causing mutation. PMID: 21866214
- CRYGD gene mutation co-segregated with all autosomal dominant congenital nuclear cataract affected individuals and was not observed in either unaffected family members or in 200 normal unrelated individuals. PMID: 21031598
- Identification of the residues in the P23T mutant that give rise to novel hydrophobic surfaces, and regions of the protein backbone where fluctuations in different timescales are restricted, which explains how lens opacity could result from this mutation. PMID: 21827768
- A novel mutation in gammaD-crystallin associated with autosomal dominant congenital cataract in a Chinese family. PMID: 21527994
- P24T mutation of gammaD-crystallin (CRYGD) was responsible for two Chinese pedigrees with congenital coralliform cataracts. CRYGD and coralliform cataracts are highly related, and P24T may be a hot-point mutation for this disorder. PMID: 21552497
- Data show that only substitutions of the second Greek key pair of each crystallin domain slowed refolding, and suggest that the second Greek keys may provide nucleation sites during the folding of the double-Greek-key crystallin domains. PMID: 21432932
- S130P point mutations of gammaD crystallin was the most resistant to aggregation, indicating a decrease of its intrinsic aggregation propensity. PMID: 21184609
- The conformational features and aggregation properties of the mutant protein E107A human gammaD-crystallin (HGDC), associated with congenital nuclear cataract, were analyzed. PMID: 21197114
- Family having anterior polar coronary cataract that co-segregates with novel allele R77S of CRYGD in all affected members. PMID: 20508808
- This work thus provides direct evidence of the dominant role played by net hydrophobic and anisotropic protein-protein interactions in the aggregation of the P23T cataract-associated gammaD-crystallin. PMID: 20553008
- Findings indicate that Glu135 and Arg142 of gammaD-crystallin are crucial for stabilizing its hydrophobic domain interface in native conformation, and disruption of charges on the gammaD-crystallin surface may lead to unfolding and subsequent aggregat'n. PMID: 19937657
- Data show that during thermal denaturation, the mutant proteins exhibited lowered thermal stability compared with WT. PMID: 19758984
- This the first report of a mutation in the Gamma-D crystallin gene (CRYGD) resulting in autosomal dominant congenital cerulean cataracts. PMID: 12676897
- This study has identified an eighth type of cataract morphology associated with CRYGD and suggests that a CRYGD mutation may underlie the historically important "coralliform" cataract first reported in 1895. PMID: 15041957
- It appearS to be caused by a missense mutation in the CRYGD gene, further supporting the notion that alterations to CRYG play an important factor in human cataract formation. PMID: 15064679
- These results suggest that insolubility, rather than loss of stability, is the primary basis for human gammaD-crystallin P23T mutation-derived congenital cataracts. PMID: 15451671
- The cataract-causing mutation proline23 to threonine does not exhibit any significant structural change relative to the native protein. However, in marked contrast to the native protein, the mutant shows a dramatically lowered solubility. PMID: 15709761
显示更多
收起更多
-
相关疾病:Cataract 4, multiple types (CTRCT4)
-
蛋白家族:Beta/gamma-crystallin family
-
数据库链接:
HGNC: 2411
OMIM: 115700
KEGG: hsa:1421
STRING: 9606.ENSP00000264376
UniGene: Hs.546247
Most popular with customers
-
Recombinant Human Claudin-18.2 (CLDN18.2)-VLPs (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Claudin-6 (CLDN6)-VLPs (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
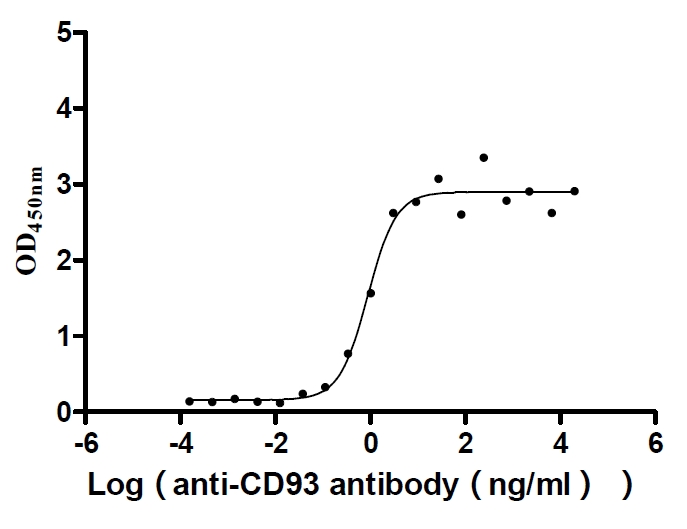
Recombinant Human Complement component C1q receptor (CD93), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Desmoglein-3 (DSG3), partial (Active)
Express system: Baculovirus
Species: Homo sapiens (Human)
-
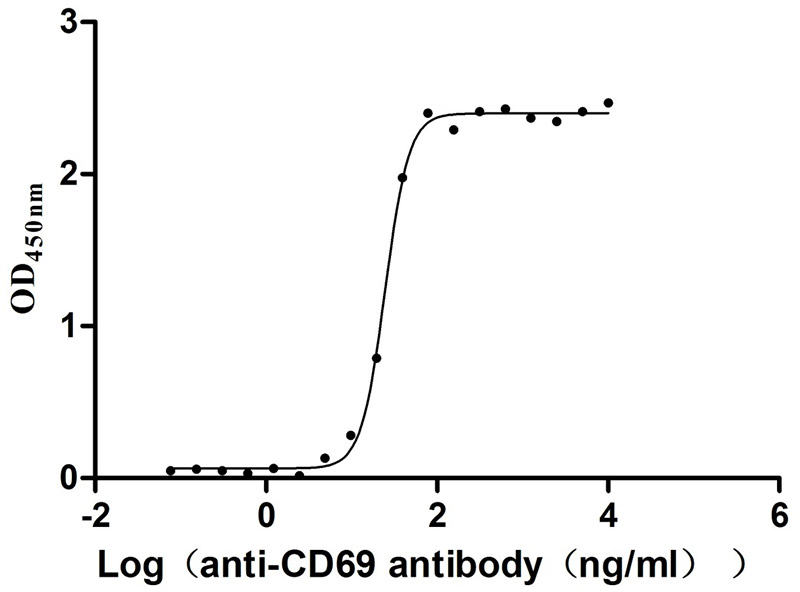
Recombinant Human Early activation antigen CD69 (CD69), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Macaca fascicularis CUB domain containing protein 1 (CDCP1), partial (Active)
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)
-
Recombinant Macaca fascicularis Transmembrane 4 L6 family member 1 (TM4SF1)-VLPs (Active)
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)
-
Express system: Mammalian cell
Species: Homo sapiens (Human)


-AC1.jpg)
-AC1.jpg)