Recombinant Human ERO1-like protein alpha (ERO1L)
-
货号:CSB-YP846631HU
-
规格:
-
来源:Yeast
-
其他:
-
货号:CSB-EP846631HU
-
规格:
-
来源:E.coli
-
其他:
-
货号:CSB-EP846631HU-B
-
规格:
-
来源:E.coli
-
共轭:Avi-tag Biotinylated
E. coli biotin ligase (BirA) is highly specific in covalently attaching biotin to the 15 amino acid AviTag peptide. This recombinant protein was biotinylated in vivo by AviTag-BirA technology, which method is BriA catalyzes amide linkage between the biotin and the specific lysine of the AviTag.
-
其他:
-
货号:CSB-BP846631HU
-
规格:
-
来源:Baculovirus
-
其他:
-
货号:CSB-MP846631HU
-
规格:
-
来源:Mammalian cell
-
其他:
产品详情
-
纯度:>85% (SDS-PAGE)
-
基因名:
-
Uniprot No.:
-
别名:Endoplasmic oxidoreductin 1 like protein; Endoplasmic oxidoreductin-1-like protein; Endoplasmic reticulum oxidoreductase 1 alpha; Endoplasmic reticulum oxidoreductin 1-like; ERO1 alpha; ERO1 L; ERO1 Lalpha; ERO1 like protein alpha; ERO1-alpha; ERO1-L; ERO1-L-alpha; ERO1-like (S. cerevisiae); ERO1-like alpha; ERO1-like protein alpha; ERO1-like; S. cerevisiae; homolog of; alpha; ERO1A; ERO1A_HUMAN; ERO1L; ERO1LA; Oxidoreductin 1 Lalpha; Oxidoreductin-1-L-alpha; PRO865; UNQ434
-
种属:Homo sapiens (Human)
-
蛋白长度:Full Length of Mature Protein
-
表达区域:24-468
-
氨基酸序列EEQPPET AAQRCFCQVS GYLDDCTCDV ETIDRFNNYR LFPRLQKLLE SDYFRYYKVN LKRPCPFWND ISQCGRRDCA VKPCQSDEVP DGIKSASYKY SEEANNLIEE CEQAERLGAV DESLSEETQK AVLQWTKHDD SSDNFCEADD IQSPEAEYVD LLLNPERYTG YKGPDAWKIW NVIYEENCFK PQTIKRPLNP LASGQGTSEE NTFYSWLEGL CVEKRAFYRL ISGLHASINV HLSARYLLQE TWLEKKWGHN ITEFQQRFDG ILTEGEGPRR LKNLYFLYLI ELRALSKVLP FFERPDFQLF TGNKIQDEEN KMLLLEILHE IKSFPLHFDE NSFFAGDKKE AHKLKEDFRL HFRNISRIMD CVGCFKCRLW GKLQTQGLGT ALKILFSEKL IANMPESGPS YEFHLTRQEI VSLFNAFGRI STSVKELENF RNLLQNIH
-
蛋白标签:Tag type will be determined during the manufacturing process.
The tag type will be determined during production process. If you have specified tag type, please tell us and we will develop the specified tag preferentially. -
产品提供形式:Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
复溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
储存条件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保质期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
货期:Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事项:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet :Please contact us to get it.
相关产品
靶点详情
-
功能:Oxidoreductase involved in disulfide bond formation in the endoplasmic reticulum. Efficiently reoxidizes P4HB/PDI, the enzyme catalyzing protein disulfide formation, in order to allow P4HB to sustain additional rounds of disulfide formation. Following P4HB reoxidation, passes its electrons to molecular oxygen via FAD, leading to the production of reactive oxygen species (ROS) in the cell. Required for the proper folding of immunoglobulins. Involved in the release of the unfolded cholera toxin from reduced P4HB/PDI in case of infection by V.cholerae, thereby playing a role in retrotranslocation of the toxin. Plays an important role in ER stress-induced, CHOP-dependent apoptosis by activating the inositol 1,4,5-trisphosphate receptor IP3R1.
-
基因功能参考文献:
- High expression of ERO1L is associated with poor prognosis of patients with gastric cancer. These results indicate that ERO1L expression may be a clinically promising therapeutic target for prevention of gastric cancer. PMID: 26987398
- ERO1alpha plays a crucial role in HSC proliferation via posttranslational modification of collagen and MT1-MMP PMID: 28774960
- Study shows that ERO1L and NARS expression level are up-regulated in primary lung adenocarcinoma and identifies them with a potential to promote tumor metastasis and growth of cancer cells. PMID: 27161446
- a mechanism of dual Ero1alpha regulation by dynamic redox interactions between PDI and the two Ero1alpha flexible loops that harbor the regulatory cysteines. PMID: 27703014
- Expression of ERO1-alpha in MDA-MB-231 cells promotes tumour growth via promoting angiogenesis. Knockdown of ERO1-alpha inhibits secretion of VEGF by inhibition of oxidative protein folding. In triple-negative breast cancer cases, the expression of ERO1-alpha was upregulated and related to the number of the blood vessels. ERO1-alpha was a poor prognosis factor in triple-negative breast cancer. PMID: 27100727
- Data indicate a new mechanism of Ero1alpha regulation in which thiol-disulfide exchange at Cys208-Cys241 affects the stability of the Cys94-Cys131 inhibitory disulfide through allosteric and/or inter-molecular communication. PMID: 26609561
- the cancer-associated ERO1-alpha regulates the expression of the MHC class I molecule via oxidative folding PMID: 25870246
- Data suggest that expression of ERO1alpha (oxidoreductin-1-L-alpha) and CHOP (c/EBP-homologous protein) is up-regulated in liver of patients with acute liver failure. PMID: 25387528
- These results suggest that overexpression of ERO1-alpha in the tumor inhibits the T cell response by recruiting polymorphonuclear myeloid-derived suppressor cells PMID: 25595776
- PDI has a role as a competent regulator and a specific substrate of Ero1alpha govern efficient and faithful oxidative protein folding and maintain the ER redox homeostasis PMID: 25258311
- These results indicate that BPA, a widely distributed and potentially harmful chemical, inhibits Ero1-PDI-mediated disulfide bond formation. PMID: 25122773
- The high expression of Ero1alpha in cancers of the esophagus and stomach demonstrates the importance of ER redox regulation in the gastro-intestinal (GI) tract in health and disease. PMID: 23373818
- GPx7 promotes oxidative protein folding, directly utilizing Ero1alpha-generated hydrogen peroxide in the early secretory compartment. PMID: 23919619
- A simple feedback mechanism of regulation of Ero1alpha was identified involving its primary substrate. PMID: 24758166
- The expression of hERO1-alpha in cancer cells is associated with poorer prognosis. PMID: 23578220
- Report on the establishment of an engineered CHOS cell line that has been engineered to express both XBP-1S) and ERO1-Lalpha and has been named CHOS-XE. CHOS-XE cells produced increased antibody (MAb) yields (5.3- 6.2 fold) in comparison to CHOS cells. PMID: 23335490
- ER stress induced by misfolded proinsulin was limited by increased expression of Ero1alpha, suggesting that enhancing the oxidative folding of proinsulin may be a viable therapeutic strategy in the treatment of type 2 diabetes. PMID: 24022479
- This paper reported the interactions of Ero1 with protein disulfide isomerase family proteins and chaperones, highlighting the effect that redox flux has on Ero1 partnerships. PMID: 23530257
- hyperoxidation generated by Ero1alpha-C104A/C131A is addressed in the ER lumen and is unlikely to exert oxidative injury throughout the cell. PMID: 23027870
- the levels, subcellular localization, and activity of Ero1alpha coordinately regulate Ca(2+) and redox homeostasis and signaling in the early secretory compartment. PMID: 21854214
- the intramolecular electron transfer from the a domain to the a' domain within PDI during its oxidation by ERO1alpha. PMID: 21757736
- Molecular bases of cyclic and specific disulfide interchange between human ERO1alpha protein and protein-disulfide isomerase (PDI). PMID: 21398518
- Results show that within the ER, Ero1alpha is almost exclusively found on the mitochondria-associated membrane (MAM). PMID: 20186508
- A dynamic equilibrium between Ero1- and glutathione disulphide-mediated oxidation of protein disulphide isomerases constitutes an important element of endoplasmic reticulum redox homeostasis. PMID: 20802462
- Ero1alpha limits its oxidative activity using four regulatory cysteines properly positioned within a critical loop that transfers electrons from protein disulphide isomerase to the FAD-containing active site. PMID: 20834232
- proving that the activity of Ero1-Lalpha results in H(2)O(2) formation in the endoplasmic reticulum PMID: 20095866
- Ero1alpha is expressed on blood platelets in association with protein-disulfide isomerase and contributes to redox-controlled remodeling of alphaIIbbeta3. PMID: 20562109
- The results demonstrate that the specificity of Ero1alpha toward the active sites of PDI requires the presence of the regulatory disulfides. PMID: 20657012
- an appropriate Ero1alpha-PDI ratio is critical for regulating the binding-release cycle of CTA1 by PDI during retro-translocation, and PDI's redox state has a role in targeting it to the retro-translocon PMID: 20130085
- oxygen regulation of ERO1-Lalpha expression likely is to maintain the transfer rate of oxidizing equivalents to PDI in situations of an altered cellular redox state induced by changes of the cellular oxygen tension PMID: 12752442
- two conserved cysteine triads in human Ero1alpha cooperate for efficient disulfide bond formation in the endoplasmic reticulum PMID: 15136577
- glutathione limits Ero1-dependent oxidation in the endoplasmic reticulum PMID: 15161913
- Hypoxic induction of Ero1-L alpha is key adaptive response in a previously unrecognized HIF-1-mediated pathway that operates to improve protein secretion under hypoxia and might be used for inhibiting tumor growth via inhibiting VEGF-driven angiogenesis. PMID: 15592500
- Results determine the redox-driven shutdown mechanism of Ero1alpha from the formation of a disulphide bond between the active-site Cys(94) and Cys(131). PMID: 18833192
- Data suggest that partial regulatory disulfide reduction may be a mechanism for preventing excessive Ero1alpha activity and oxidation of PDI or that additional factors are required for Ero1alpha activation within the mammalian ER. PMID: 18971943
- In this report, the structural model of human Ero1-L alpha was built at first. PMID: 19766098
显示更多
收起更多
-
亚细胞定位:Endoplasmic reticulum membrane; Peripheral membrane protein; Lumenal side. Note=The association with ERP44 is essential for its retention in the endoplasmic reticulum.
-
蛋白家族:EROs family
-
组织特异性:Widely expressed at low level. Expressed at high level in upper digestive tract. Highly expressed in esophagus. Weakly expressed in stomach and duodenum.
-
数据库链接:
HGNC: 13280
OMIM: 615435
KEGG: hsa:30001
STRING: 9606.ENSP00000379042
UniGene: Hs.525339
Most popular with customers
-
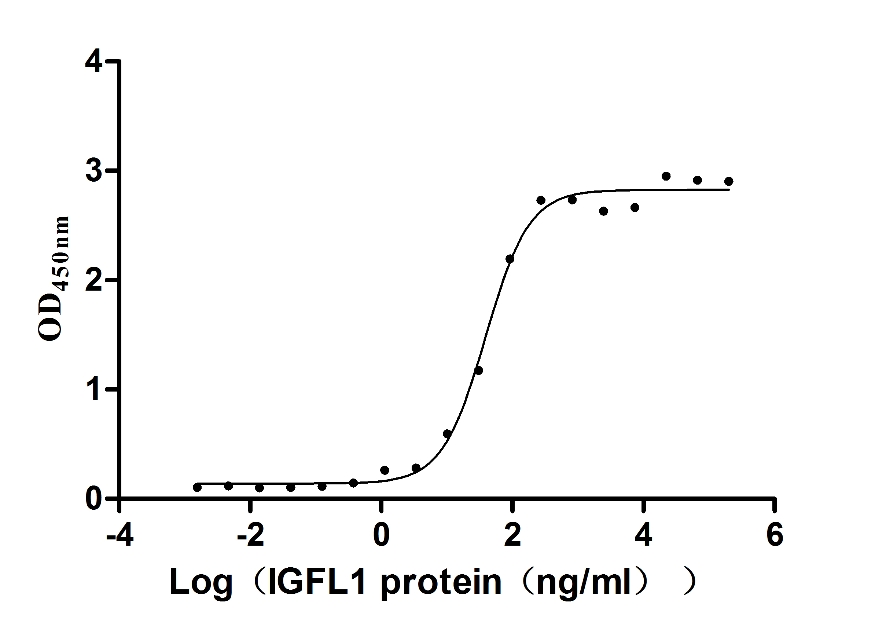
Recombinant Human Insulin growth factor-like family member 1 (IGFL1) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Angiopoietin-2 (ANGPT2) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
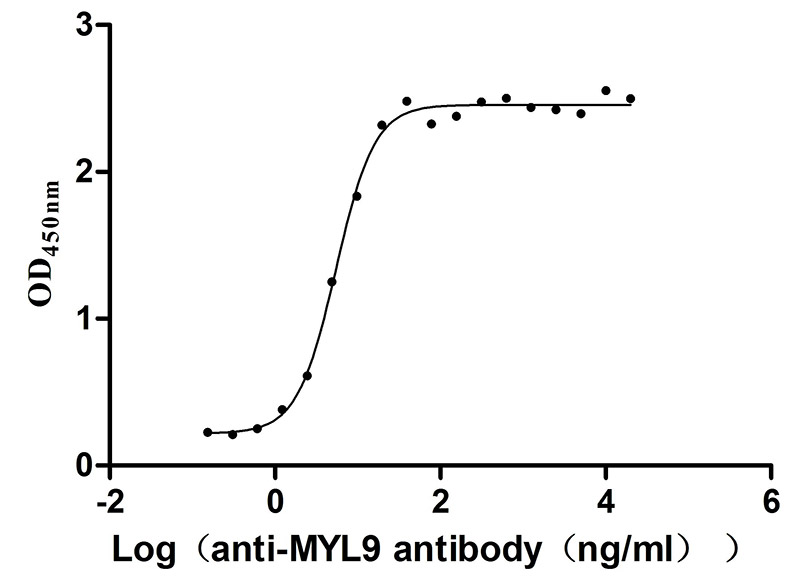
Recombinant Human Myosin regulatory light polypeptide 9 (MYL9) (Active)
Express system: Yeast
Species: Homo sapiens (Human)
-
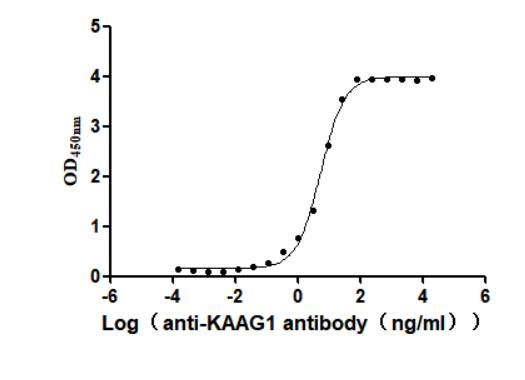
Recombinant Human Kidney-associated antigen 1(KAAG1) (Active)
Express system: Baculovirus
Species: Homo sapiens (Human)
-
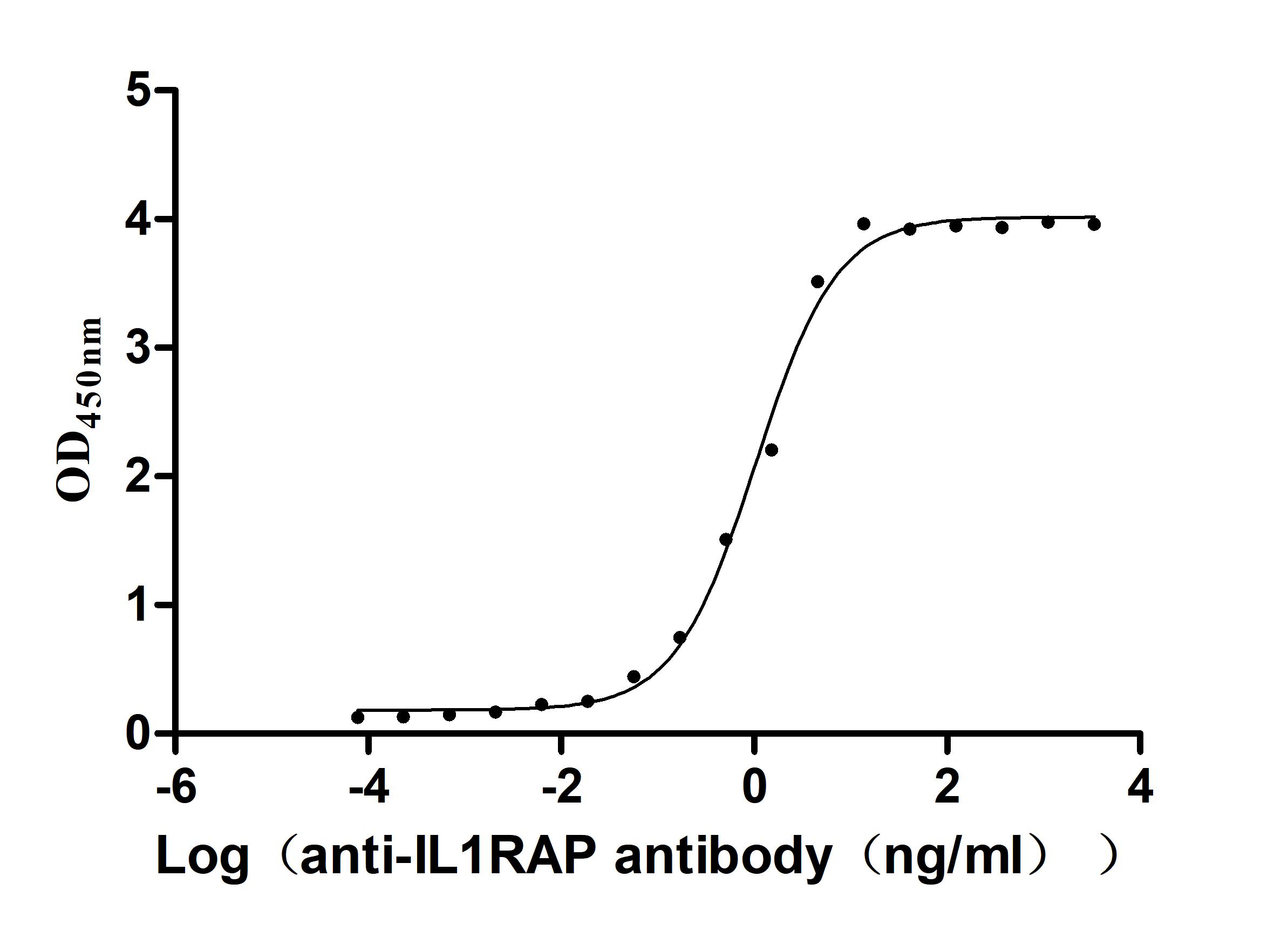
Recombinant Macaca fascicularis Interleukin 1 receptor accessory protein(IL1RAP), partial (Active)
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)


-AC1.jpg)