Recombinant Human E3 ubiquitin-protein ligase TRIP12 (TRIP12), partial
-
货号:CSB-YP617924HU
-
规格:
-
来源:Yeast
-
其他:
-
货号:CSB-EP617924HU-B
-
规格:
-
来源:E.coli
-
共轭:Avi-tag Biotinylated
E. coli biotin ligase (BirA) is highly specific in covalently attaching biotin to the 15 amino acid AviTag peptide. This recombinant protein was biotinylated in vivo by AviTag-BirA technology, which method is BriA catalyzes amide linkage between the biotin and the specific lysine of the AviTag.
-
其他:
-
货号:CSB-MP617924HU
-
规格:
-
来源:Mammalian cell
-
其他:
产品详情
-
纯度:>85% (SDS-PAGE)
-
基因名:
-
Uniprot No.:
-
别名:TRIP12; KIAA0045; ULF; E3 ubiquitin-protein ligase TRIP12; EC 2.3.2.26; E3 ubiquitin-protein ligase for Arf; ULF; HECT-type E3 ubiquitin transferase TRIP12; Thyroid receptor-interacting protein 12; TR-interacting protein 12; TRIP-12
-
种属:Homo sapiens (Human)
-
蛋白长度:Partial
-
蛋白标签:Tag type will be determined during the manufacturing process.
The tag type will be determined during production process. If you have specified tag type, please tell us and we will develop the specified tag preferentially. -
产品提供形式:Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
复溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
储存条件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保质期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
货期:Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事项:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet :Please contact us to get it.
相关产品
靶点详情
-
功能:E3 ubiquitin-protein ligase involved in ubiquitin fusion degradation (UFD) pathway and regulation of DNA repair. Part of the ubiquitin fusion degradation (UFD) pathway, a process that mediates ubiquitination of protein at their N-terminus, regardless of the presence of lysine residues in target proteins. Acts as a key regulator of DNA damage response by acting as a suppressor of RNF168, an E3 ubiquitin-protein ligase that promotes accumulation of 'Lys-63'-linked histone H2A and H2AX at DNA damage sites, thereby acting as a guard against excessive spreading of ubiquitinated chromatin at damaged chromosomes. In normal cells, mediates ubiquitination and degradation of isoform p19ARF/ARF of CDKN2A, a lysine-less tumor suppressor required for p53/TP53 activation under oncogenic stress. In cancer cells, however, isoform p19ARF/ARF and TRIP12 are located in different cell compartments, preventing isoform p19ARF/ARF ubiquitination and degradation. Does not mediate ubiquitination of isoform p16-INK4a of CDKN2A. Also catalyzes ubiquitination of NAE1 and SMARCE1, leading to their degradation. Ubiquitination and degradation of target proteins is regulated by interaction with proteins such as MYC, TRADD or SMARCC1, which disrupt the interaction between TRIP12 and target proteins. Mediates ubiquitination of ASXL1: following binding to N(6)-methyladenosine methylated DNA, ASXL1 is ubiquitinated by TRIP12, leading to its degradation and subsequent inactivation of the PR-DUB complex.
-
基因功能参考文献:
- p16 overexpression led to downregulation of TRIP12, which in turn led to increased RNF168 levels, repressed DNA damage repair (DDR), increased 53BP1 foci and enhanced radioresponsiveness. PMID: 27425591
- We describe the TRIP12-associated phenotype, showing that TRIP12 is a risk gene for non-syndromic intellectual disability with and without autism spectrum disorder, and that TRIP12 mutation carriers present with a broad phenotypic range within the neurodevelopmental phenotypes. PMID: 27848077
- nine presented pathogenic variants further document that TRIP12 haploinsufficiency causes a childhood-onset neurodevelopmental disorder PMID: 28251352
- Data indicate that E3 ubiquitin ligase thyroid hormone receptor-interacting protein 12 (TRIP12) promotes proteasomal degradation of pancreas transcription factor 1a (PTF1a)and regulates PTF1a activities. PMID: 25355311
- An exon3-skipping event in TRIP12 was detected in acute myeloid leukemia patients at remission PMID: 24961348
- HUWE1 and TRIP12 collaborate in degradation of ubiquitin-fusion proteins and misframed ubiquitin. PMID: 23209776
- Study shows that TRIP12 and UBR5, two HECT domain ubiquitin E3 ligases, control accumulation of RNF168, a rate-limiting component of a pathway that ubiquitylates histones after DNA breakage. PMID: 22884692
- data indicate that TRADD shuttles dynamically from the cytoplasm into the nucleus to modulate the interaction between p19(Arf) and its E3 ubiquitin ligase ULF, thereby promoting p19(Arf) protein stability and tumour suppression PMID: 22561347
- ULF is a bona fide E3 ligase for ARF and also suggest that ULF is an important target for activating the ARF-p53 axis in human acute myeloid leukaemia cells. PMID: 20699639
- Data show that the mechanism of BAF155-mediated stabilization of BAF57 involves blocking its ubiquitination by preventing interaction with TRIP12. PMID: 20829358
- TRIP12 promotes degradation of APP-BP1 by catalyzing its ubiquitination, which in turn modulates the neddylation pathway. PMID: 18627766
- The HECT domain of TRIP12 ubiquitinates substrates of the ubiquitin fusion degradation pathway. PMID: 19028681
显示更多
收起更多
-
亚细胞定位:Nucleus, nucleoplasm.
-
蛋白家族:UPL family, K-HECT subfamily
-
数据库链接:
HGNC: 12306
OMIM: 604506
KEGG: hsa:9320
STRING: 9606.ENSP00000283943
UniGene: Hs.572642
Most popular with customers
-
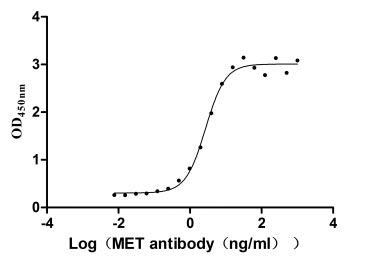
Recombinant Human Hepatocyte growth factor receptor (MET), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Mouse GDNF family receptor alpha-like (Gfral), partial (Active)
Express system: Mammalian cell
Species: Mus musculus (Mouse)
-
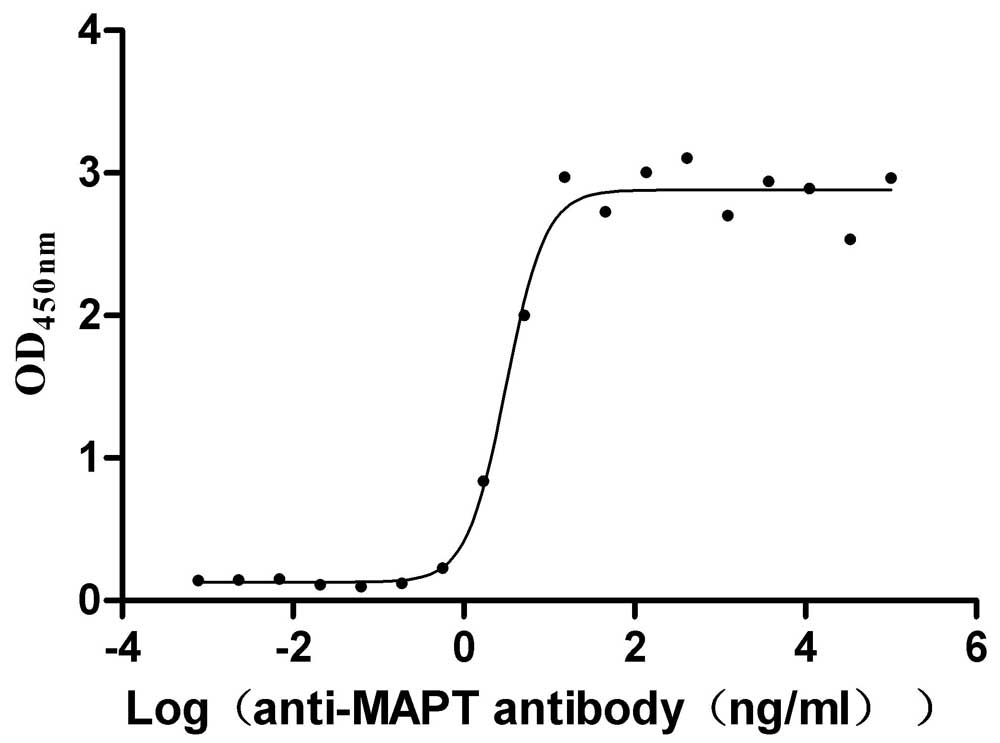
Recombinant Macaca mulatta Microtubule-associated protein tau (MAPT) (Active)
Express system: Mammalian cell
Species: Macaca mulatta (Rhesus macaque)
-
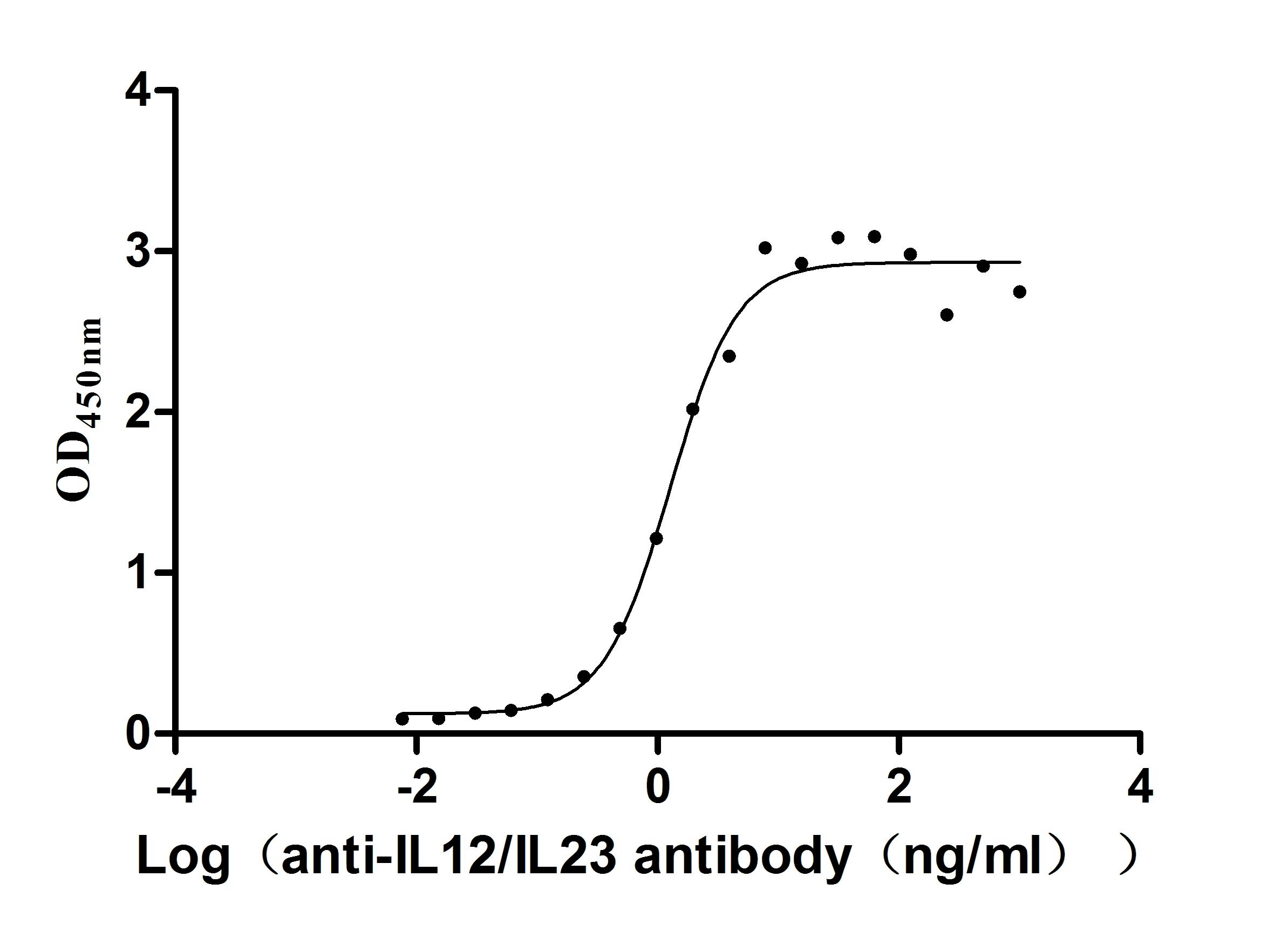
Recombinant Human IL12B&IL12A Heterodimer Protein (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Mouse Cell adhesion molecule 1 (Cadm1), partial (Active)
Express system: Mammalian cell
Species: Mus musculus (Mouse)
-
Recombinant Human Alkaline phosphatase, germ cell type (ALPG) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Transferrin receptor protein 1 (TFRC), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Cadherin-17 (CDH17), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)