Recombinant Human Centrin-2 (CETN2)
-
货号:CSB-YP005265HU
-
规格:
-
来源:Yeast
-
其他:
-
货号:CSB-EP005265HU-B
-
规格:
-
来源:E.coli
-
共轭:Avi-tag Biotinylated
E. coli biotin ligase (BirA) is highly specific in covalently attaching biotin to the 15 amino acid AviTag peptide. This recombinant protein was biotinylated in vivo by AviTag-BirA technology, which method is BriA catalyzes amide linkage between the biotin and the specific lysine of the AviTag.
-
其他:
-
货号:CSB-BP005265HU
-
规格:
-
来源:Baculovirus
-
其他:
-
货号:CSB-MP005265HU
-
规格:
-
来源:Mammalian cell
-
其他:
产品详情
-
纯度:>85% (SDS-PAGE)
-
基因名:
-
Uniprot No.:
-
别名:20kD calcium binding protein; CALT; caltractin; Caltractin isoform 1; CEN2; centrin; centrin; EF hand protein; 2 ; Centrin-2; Centrin2; CETN2; CETN2_HUMAN; EF hand protein 2; EF-hand protein
-
种属:Homo sapiens (Human)
-
蛋白长度:Full Length of Mature Protein
-
表达区域:2-172
-
氨基酸序列ASNFKKANM ASSSQRKRMS PKPELTEEQK QEIREAFDLF DADGTGTIDV KELKVAMRAL GFEPKKEEIK KMISEIDKEG TGKMNFGDFL TVMTQKMSEK DTKEEILKAF KLFDDDETGK ISFKNLKRVA KELGENLTDE ELQEMIDEAD RDGDGEVSEQ EFLRIMKKTS LY
-
蛋白标签:Tag type will be determined during the manufacturing process.
The tag type will be determined during production process. If you have specified tag type, please tell us and we will develop the specified tag preferentially. -
产品提供形式:Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
复溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
储存条件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保质期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
货期:Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事项:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet :Please contact us to get it.
相关产品
靶点详情
-
功能:Plays a fundamental role in microtubule organizing center structure and function. Required for centriole duplication and correct spindle formation. Has a role in regulating cytokinesis and genome stability via cooperation with CALM1 and CCP110.; Involved in global genome nucleotide excision repair (GG-NER) by acting as component of the XPC complex. Cooperatively with RAD23B appears to stabilize XPC. In vitro, stimulates DNA binding of the XPC:RAD23B dimer.; The XPC complex is proposed to represent the first factor bound at the sites of DNA damage and together with other core recognition factors, XPA, RPA and the TFIIH complex, is part of the pre-incision (or initial recognition) complex. The XPC complex recognizes a wide spectrum of damaged DNA characterized by distortions of the DNA helix such as single-stranded loops, mismatched bubbles or single-stranded overhangs. The orientation of XPC complex binding appears to be crucial for inducing a productive NER. XPC complex is proposed to recognize and to interact with unpaired bases on the undamaged DNA strand which is followed by recruitment of the TFIIH complex and subsequent scanning for lesions in the opposite strand in a 5'-to-3' direction by the NER machinery. Cyclobutane pyrimidine dimers (CPDs) which are formed upon UV-induced DNA damage esacpe detection by the XPC complex due to a low degree of structural perurbation. Instead they are detected by the UV-DDB complex which in turn recruits and cooperates with the XPC complex in the respective DNA repair.; As a component of the TREX-2 complex, involved in the export of mRNAs to the cytoplasm through the nuclear pores.
-
基因功能参考文献:
- Multidisciplinary approach showed that HsPrp40Ap interacts with centrin in vitro, supporting a coupled functional role for these proteins in pre-mRNA splicing. PMID: 28636910
- Cetn3 inhibits Mps1 autophosphorylation at Thr-676, a known site of T-loop autoactivation, and interferes with Mps1-dependent phosphorylation of Cetn2. The cellular overexpression of Cetn3 attenuates the incorporation of Cetn2 into centrioles and centrosome reduplication, whereas depletion of Cetn3 generates extra centrioles. PMID: 26354417
- Centrin2 regulates primary ciliogenesis through controlling CP110 levels. PMID: 25753040
- co-depletion of centrin 2 and PCID2 leads to blocking rather than delaying nuclear protein export, indicating the dominance of the centrin 2 phenotype. PMID: 24291146
- Data indicate that overexpression of the centrin interactor POC5 leads to the assembly of linear, centrin-dependent structures. PMID: 23844208
- Cen2 influences the binding of RPA and XPA with damaged DNA. PMID: 22809153
- xeroderma pigmentosum complementation group C expression correlates with a decreased amount of CENTRIN 2 transcript and protein PMID: 21676658
- The stability of centrin is regulated in part by Aurora A. PMID: 21731694
- Mps1-dependent phosphorylation of Cetn2 stimulates the canonical centriole assembly pathway. PMID: 20980622
- oxidative radicals induce high proportions of irreversible damages (polymerisation) centrin 2 is highly sensitive to ionising radiation. PMID: 20586543
- required for centriole duplication in mammalian cells PMID: 12176356
- The solution structure of the long C-terminal fragment of centrin 2 exhibits an open two EF-hand structure, similar to the conformation of related Ca(2+)-saturated regulatory domains. PMID: 12578356
- structural characterization of the complex formed by the C-terminal domain of Cen2 with a peptide of xeroderma pigmentosum group C protein PMID: 12890685
- Results describe the self-assembly properties of purified human centrin-2 in vitro. PMID: 15356003
- Centrin 2 stimulates nucleotide excision repair by interacting with XPC. PMID: 15964821
- an 18-residue peptide, from the N-terminal unstructured fragment, has a significant affinity for the isolated C-terminal domain, suggesting an active role in the self-assembly of centrin molecules. PMID: 16411764
- the crystal structure of calcium-loaded full-length centrin-2 complexed with a xeroderma pigmentosum group C peptide; a novel binding motif for centrin PMID: 16627479
- A complex formed by a Ca2+-bound human centrin 2 with a 17-mer peptide derived from the XPC sequence was crystallized. PMID: 16820684
- Centrin 2 is highly sensitive to ionizing radiation, which could have important consequences on its biological functions. PMID: 17603931
- The present data confirm that the in vitro structural features of the centrin/XPC peptide complex are highly relevant to the cellular context. PMID: 17897675
- CETN2 localizes to the vertebrate nuclear pore and plays a role in mRNA and protein export. PMID: 18172010
- NMR analysis indicates that the physical interaction between C-XPC and centrin 2 induces only minor conformational changes into XPC, localized around the 17-mer segment (847-863), showed to be critically involved in the centrin binding. PMID: 18177054
- lower centrin levels in oligoasthnozoospermic males resulted in lower pregnancy percentage in this group after ICSI. PMID: 19179680
- The nucleocytoplasmic shuttling of centrin-2 depends on the SUMO system. PMID: 19706679
- The structure of C-HsCen2 [the C-terminal domain of HsCen2 (T94-Y172)] in complex with R17-hSfi1-20 was determined. PMID: 19857500
显示更多
收起更多
-
亚细胞定位:Cytoplasm, cytoskeleton, microtubule organizing center, centrosome. Cytoplasm, cytoskeleton, microtubule organizing center, centrosome, centriole. Nucleus envelope. Nucleus, nuclear pore complex. Nucleus.
-
蛋白家族:Centrin family
-
数据库链接:
HGNC: 1867
OMIM: 300006
KEGG: hsa:1069
STRING: 9606.ENSP00000359300
UniGene: Hs.82794
Most popular with customers
-
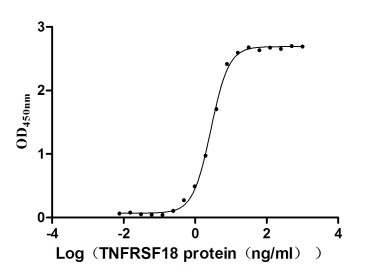
Recombinant Human Tumor necrosis factor receptor superfamily member 18 (TNFRSF18), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
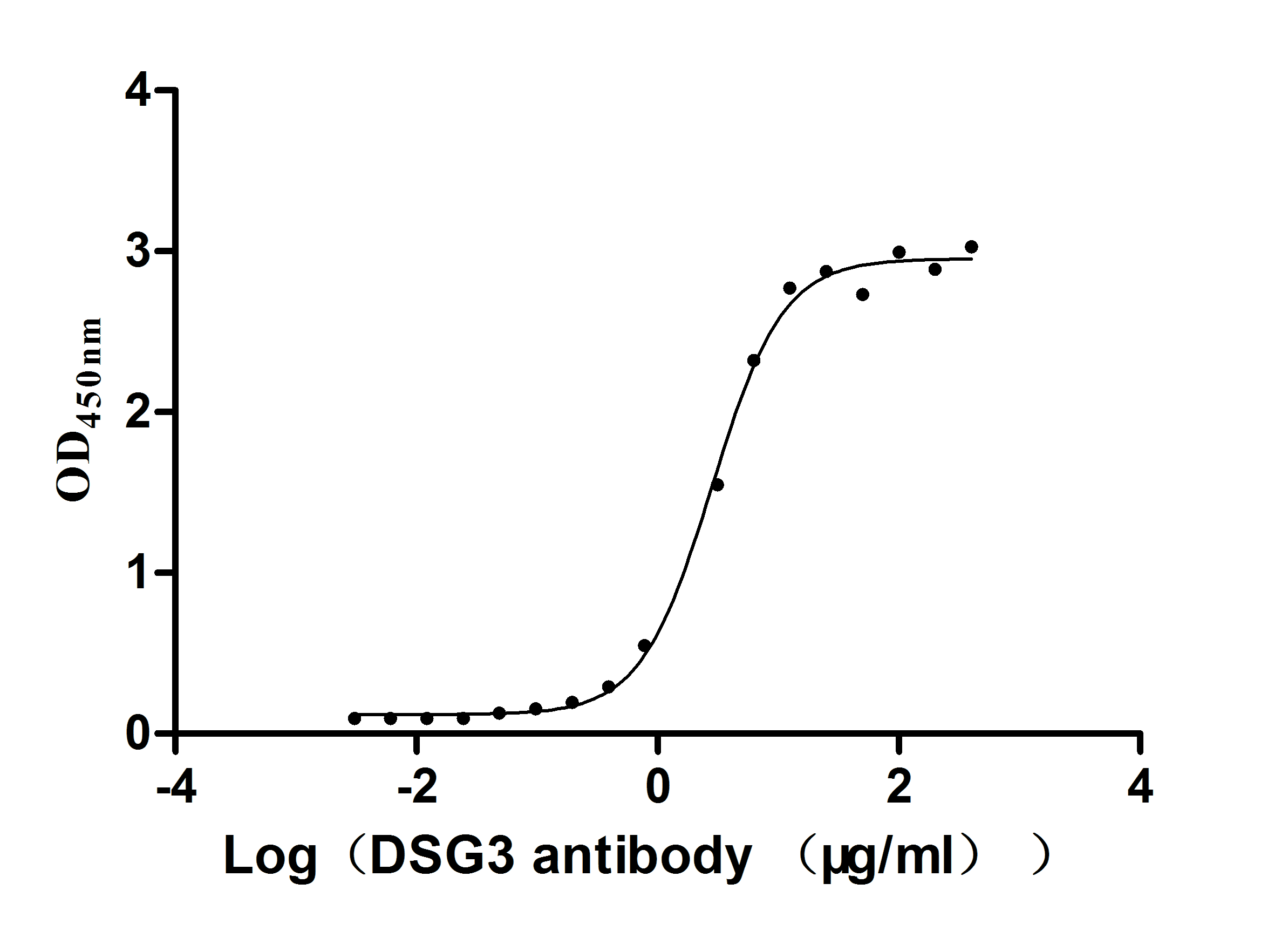
Recombinant Mouse Desmoglein-3 (Dsg3), partial (Active)
Express system: Mammalian cell
Species: Mus musculus (Mouse)
-
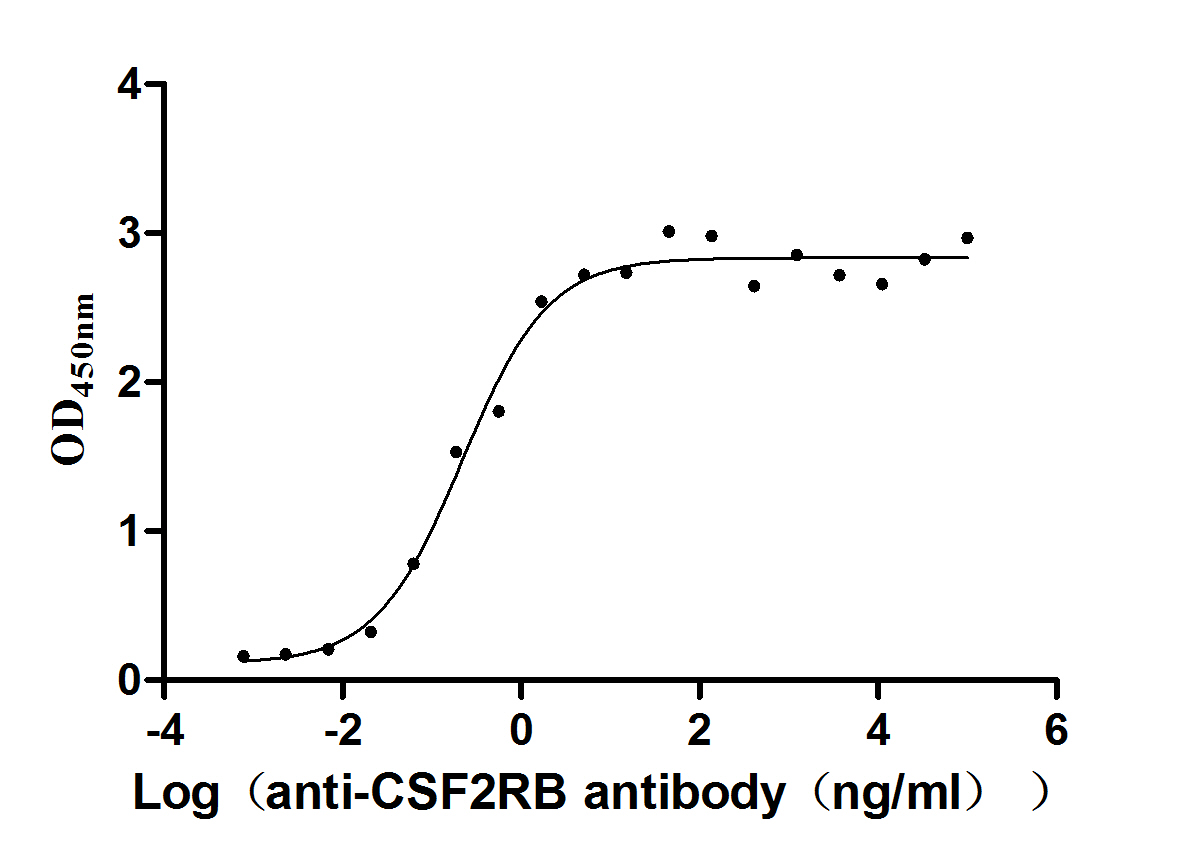
Recombinant Human Cytokine receptor common subunit beta (CSF2RB), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
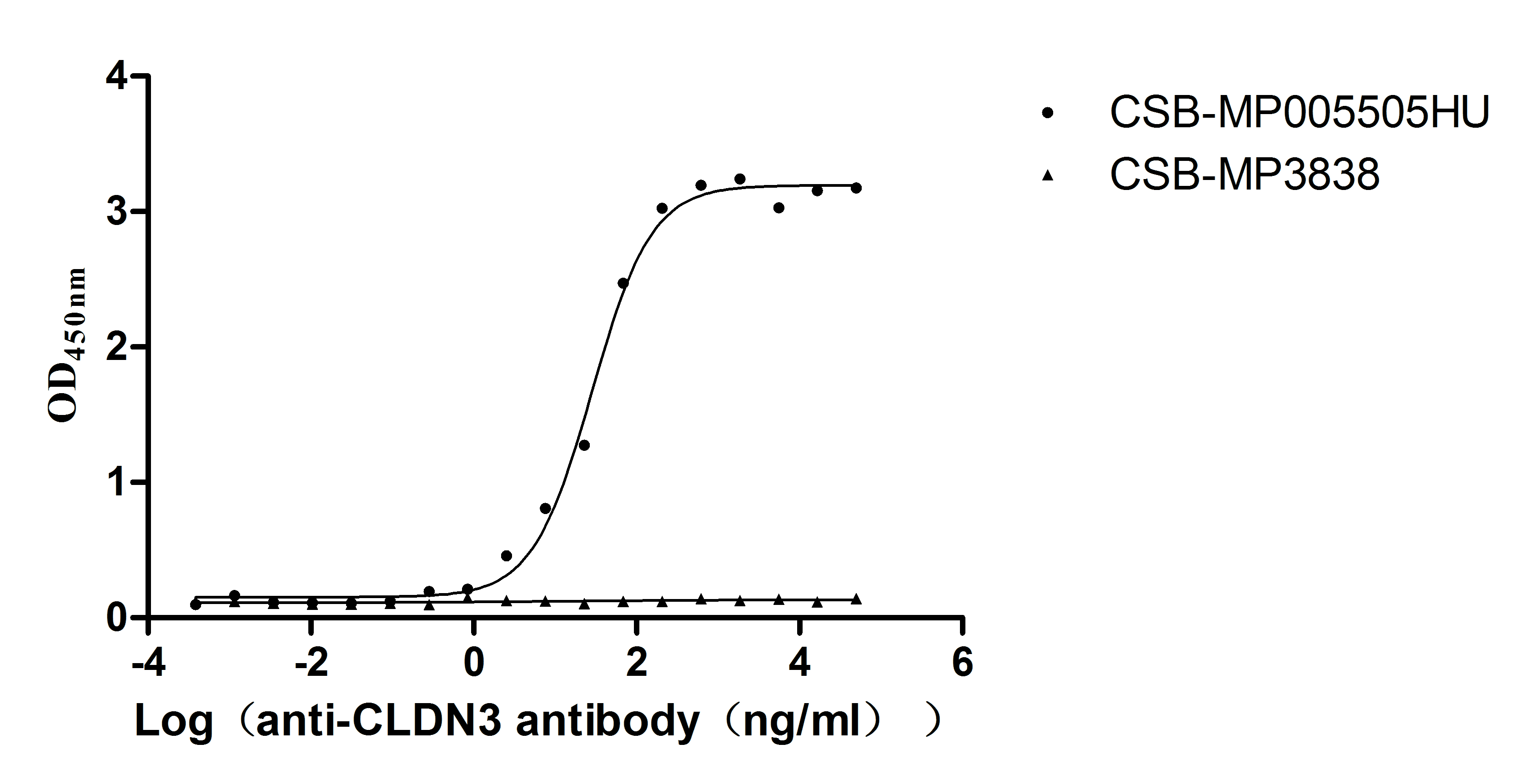
Recombinant Human Claudin-3 (CLDN3)-VLPs (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Tumor-associated calcium signal transducer 2 (TACSTD2), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Interleukin-2 (IL2) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Macaca fascicularis CUB domain containing protein 1 (CDCP1), partial (Active)
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)
-
Recombinant Macaca fascicularis C-type lectin domain family 4 member C(CLEC4C), partial (Active)
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)