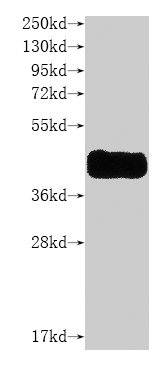
Recombinant Bovine Rhodopsin (RHO), partial
In Stock Promotion-
货号:CSB-EP019681BO1
-
规格:¥2328
-
促销:

-
图片:
-
其他:
产品详情
-
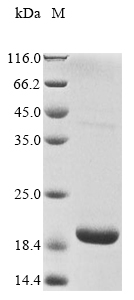
纯度:Greater than 85% as determined by SDS-PAGE.
-
基因名:
-
Uniprot No.:
-
别名:RHO; Rhodopsin
-
种属:Bos taurus (Bovine)
-
蛋白长度:Partial
-
来源:E.coli
-
分子量:19.5 kDa
-
表达区域:1-36aa
-
氨基酸序列MNGTEGPNFYVPFSNKTGVVRSPFEAPQYYLAEPWQ
Note: The complete sequence including tag sequence, target protein sequence and linker sequence could be provided upon request. -
蛋白标签:N-terminal 6xHis-KSI-tagged
-
产品提供形式:Liquid or Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
缓冲液:If the delivery form is liquid, the default storage buffer is Tris/PBS-based buffer, 5%-50% glycerol. If the delivery form is lyophilized powder, the buffer before lyophilization is Tris/PBS-based buffer, 6% Trehalose, pH 8.0.
-
复溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20°C/-80°C. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
储存条件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保质期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
货期:3-7 business days
-
注意事项:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet & COA:Please contact us to get it.
相关产品
靶点详情
-
功能:Photoreceptor required for image-forming vision at low light intensity. Required for photoreceptor cell viability after birth. Light-induced isomerization of 11-cis to all-trans retinal triggers a conformational change that activates signaling via G-proteins. Subsequent receptor phosphorylation mediates displacement of the bound G-protein alpha subunit by the arrestin SAG and terminates signaling.
-
基因功能参考文献:
- Data indicate molecular dynamics simulations and site-directed fluorescence experiments on arrestin-1 interactions with rhodopsin, showing that loops within the C-edge of arrestin function as a membrane anchor. PMID: 28220785
- the photoreceptor pathology associated with expression of these enigmatic Retinitis pigmentosa-associated rhodopsin pigments arises from their unexpected inability to dimerize via transmembrane helices 1 and 5 PMID: 27694816
- Rhodopsin mutant E113Q could have the potential for use as a template of anion biosensors at visible wavelength. PMID: 27865136
- The study shows that, compared to the inactive 11-cis-retinal case, trans-retinal rhodopsin is able to undergo protonated Schiff base (PSB) deprotonation due to a change in the conformation of the retinal and a consequent alteration in the hydrogen-bond (HB) network in which PSB and the counterion Glu113 are embedded. PMID: 28731695
- Data suggest that retinitis pigmentosa-associated mutation G51A behaves differently in human rhodopsin compared to bovine rhodopsin; human rhodopsin is more thermally stable than ancestral ancestrally reconstructed mammalian rhodopsin. PMID: 28369862
- These findings revealed a total water flux between the bulk and the protein inside in the Meta II state, and suggested that these pathways provide water molecules to the crucial sites of the activated rhodopsin. PMID: 28493967
- Data suggest that a hetero-multimer complex forms between light-activated rhodopsin and light-activated heterotrimeric transducin (T-alpha-1, Gnb1, Gngt1); the stoichiometry is 1:1 rhodopsin:transducin. The complex appears to form on native rod outer segment membranes upon light activation. PMID: 28655769
- Study presents a comprehensive analysis of the kinetics and thermodynamics of the recombination reaction between opsin and 11-cis-retinal (11CR) to form the mature visual pigment, Rho; and found that the lipid bilayer environment is important for ligand binding in Rho. PMID: 28700926
- In response to light-induced isomerization of the retinal chromophore rhodopsin, hydrogen-bonding interactions involving these C=O groups are released, thus facilitating repacking of H5 and H7 onto the transmembrane core of the receptor. PMID: 27376589
- rhodopsin can tolerate a second Lys in the retinal binding pocket and suggest that an evolutionary intermediate with two Lys could allow migration of the Schiff base Lys to a position other than the observed, highly conserved location in the seventh TM helix PMID: 27486845
- multiconfigurational quantum chemistry is used to compare the isomerization mechanisms of the sensory rhodopsin from the cyanobacterium Anabaena PCC 7120 (ASR) and of the bovine rhodopsin (Rh). PMID: 26607446
- show that although the basic activation pathways of human and bovine rhodopsin are similar, structural deviations exist in the inactive conformation and during receptor activation, even between closely related rhodopsins PMID: 26105054
- Data suggest that, upon activation/deactivation of RHO, the main conformational changes found in molecular dynamic simulations are distributed throughout transmembrane bundle rather than localized to specific sites (i.e., conserved sequences). PMID: 24889093
- DMPC/DHPC bicelles dramatically increase the thermal stability of the rhodopsin mutants G90V and N55K. PMID: 26181234
- The molecular mechanism of the ultrafast reversible photoreaction of visual pigment rhodopsin may be used as a concept for the development of an ultrafast optical molecular switch. PMID: 25393597
- Formation and decay of the arrestin.rhodopsin complex in native disc membranes. PMID: 25847250
- Phospholipid scrambling is a constitutive activity of rhodopsin, distinct from its light-sensing function. PMID: 25296113
- maps of information flow were calculated in A2 A adenosine receptor (A2 A AR) and bovine rhodopsin and identified key residues for signal transductions and their pathways. PMID: 24166702
- One site on rhodopsin can interact with multiple structurally separate sites on arrestin that are almost 30 angstroms apart. PMID: 24724832
- A comparison of melanopsin with the mechanisms documented for vertebrate (bovine) and invertebrate (squid) visual photoreceptors shows that such a mechanism is not affected by the diversity of the three chromophore cavities. PMID: 24449866
- Retinitis pigmentosa mutants provide insight into the role of the N-terminal cap in rhodopsin folding, structure, and function. PMID: 24106275
- The purpose of this study was to test for mechanisms by which the autosomal dominant rhodopsin mutation Ter349Glu causes an early, rapid retinal degeneration. PMID: 23940033
- findings show that a quantum chemical model of rhodopsin provides a molecular-level understanding of the Barlow correlation; the transition state mediating thermal activation has the same electronic structure as the photoreceptor excited state, creating a direct link between maximum absorption wavelength and thermal activation kinetic constant PMID: 22955833
- Results describe the geometries, electronic effects, and vertical excitation energies in the dark state of mutated human and cattle rhodopsins carrying the abnormal substitutions M207R or S186W at the retinal binding pocket. PMID: 22126625
- The effects of inorganic salts on the thermal decay properties of both its inactive and photoactivated states on the alpha-helical membrane protein rhodopsin from vertebrate retina, is reported. PMID: 22261069
- Expression of the human RHO P23H transgene in the retina creates a miniature swine model with an inheritance pattern and retinal function that mimics adRP. PMID: 22247487
- The glycosylation pattern in the serotonin receptor (5-HT4R) is more complex than in murine and bovine rhodopsin. PMID: 22145929
- The M257Y(6.40) constitutively active mutant of the photoreceptor rhodopsin was used in combination with the specific binding of a C-terminal fragment from the G protein alpha subunit (GalphaCT) to trap a light activated state for crystallization. PMID: 22198838
- Rhodopsin/transducin complex forms only if rhodopsin is in the activated state. PMID: 21995315
- Conformational dynamics of helix 8 in the GPCR rhodopsin controls arrestin activation in the desensitization process PMID: 22039220
- kinetic analysis of thermal decay of rhodopsin reveals unusual energetics of thermal isomerization and hydrolysis of Schiff base PMID: 21921035
- Molecular mechanisms of disease for mutations at Gly-90 in rhodopsin. PMID: 21940625
- These results indicate the bilayer structure increases the activation energy of denaturation to rhodopsin denaturation. PMID: 21689528
- in the presence of antibodies against immune-dominant epitopes, recoverin loses its ability to perform a function of Ca(2+)-sensitive inhibitor of rhodopsin phosphorylation PMID: 21568868
- Transfection of hTERT-RPE1 cells with constructs encoding RHO-EGFP, but not RHO-mCherry, results in the distribution of fluorescently-tagged opsin in the plasma membrane. PMID: 20238016
- Role of bulk water in hydrolysis of the rhodopsin chromophore. PMID: 21460218
- multiscale activation mechanism with a complex energy landscape, whereby the photonic energy is directed against the E2 loop by the C13-methyl group, and toward helices H3 and H5 by the C5-methyl of the beta-ionone ring. PMID: 21527723
- Solid-state (2)H NMR relaxation elucidates picosecond-to-nanosecond-timescale motions of the retinal ligand that influence larger-scale functional dynamics of rhodopsin in membranes. PMID: 21278756
- We hypothesize that, although arrestin requires at least a single Rho*P to bind the membrane, a single arrestin can actually interact with a pair of receptors PMID: 21169358
- Analyses of the photobleaching processes of split rhodopsins and rhodopsin mutant lacking disulfide bond showed that the rigid structure of second extracellular loop is required for facilitating the formation of the active state. PMID: 20886156
- similar to transducin activation, rhodopsin phosphorylation by GRK1 and high affinity arrestin-1 binding only requires a rhodopsin monomer PMID: 20966068
- hydrophobic interactions by V138(3).(3), V227.(2), V250.(3)(3), V254.(3) and I255.(3) are critical for receptor activation and/or efficient rhodopsin-transducin interaction PMID: 21114958
- These data suggest that a larger conformational change in helices V and VI of bovine rhodopsin explains why it has greater G protein activation ability than other rhodopsins. PMID: 19497849
- Specific binding of rhodopsin-loaded nanoscale apolipoprotein bound bilayer particles to the surface and formation of a membrane protein monolayer. PMID: 20923668
- Both blue- and green-sensitive rod rhodopsins have at least one allosteric binding site for retinoid, but beta-ionone binds to the latter type of rhodopsin with low affinity and low efficacy. PMID: 20923672
- The amino acid residues that differ naturally between bovine and mouse rhodopsin appear to have minimal bearing on molecular interactions stabilizing structural segments and unfolding intermediates; no major differences in unfolding energy are observed. PMID: 21038881
- results indicate that the counterion does not need to be located at position 113 for a high photosensitivity for natural light. result suggests that counterion in vertebrate visual pigments is optimally located for stability of the Schiff base linkage. PMID: 21038858
- The crystal structure of opsin in the region of the ionic lock reflects the active state of the receptor. PMID: 21041664
- The experiments point to the importance of interactions of rhodopsin with particular lipid species in the first layer of lipids surrounding the protein as well as to membrane elastic stress in the lipid-protein domain. PMID: 20682259
- ultrafast optical spectroscopy with sub-20-fs time resolution and spectral coverage from the visible to the near-infrared allows following of the dynamics leading to the conical intersection in rhodopsin isomerization PMID: 20864998
收起更多
-
亚细胞定位:Membrane; Multi-pass membrane protein. Cell projection, cilium, photoreceptor outer segment.
-
蛋白家族:G-protein coupled receptor 1 family, Opsin subfamily
-
组织特异性:Expressed in rod-shaped photoreceptor cells in the retina that mediate vision in dim light (at protein level).
-
数据库链接:
KEGG: bta:509933
STRING: 9913.ENSBTAP00000001730
UniGene: Bt.12753