TRAIP Antibody
-
货号:CSB-PA697715
-
规格:¥1100
-
图片:
-
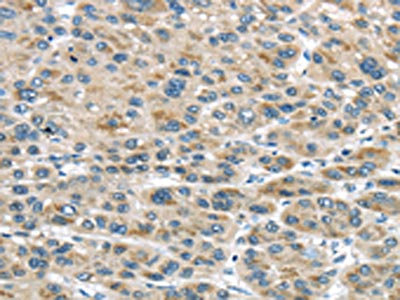
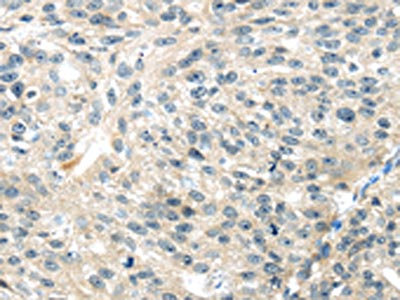
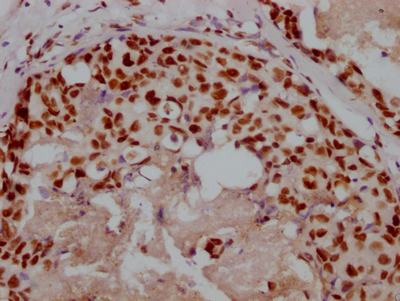
The image on the left is immunohistochemistry of paraffin-embedded Human liver cancer tissue using CSB-PA697715(TRAIP Antibody) at dilution 1/40, on the right is treated with fusion protein. (Original magnification: ×200)
-
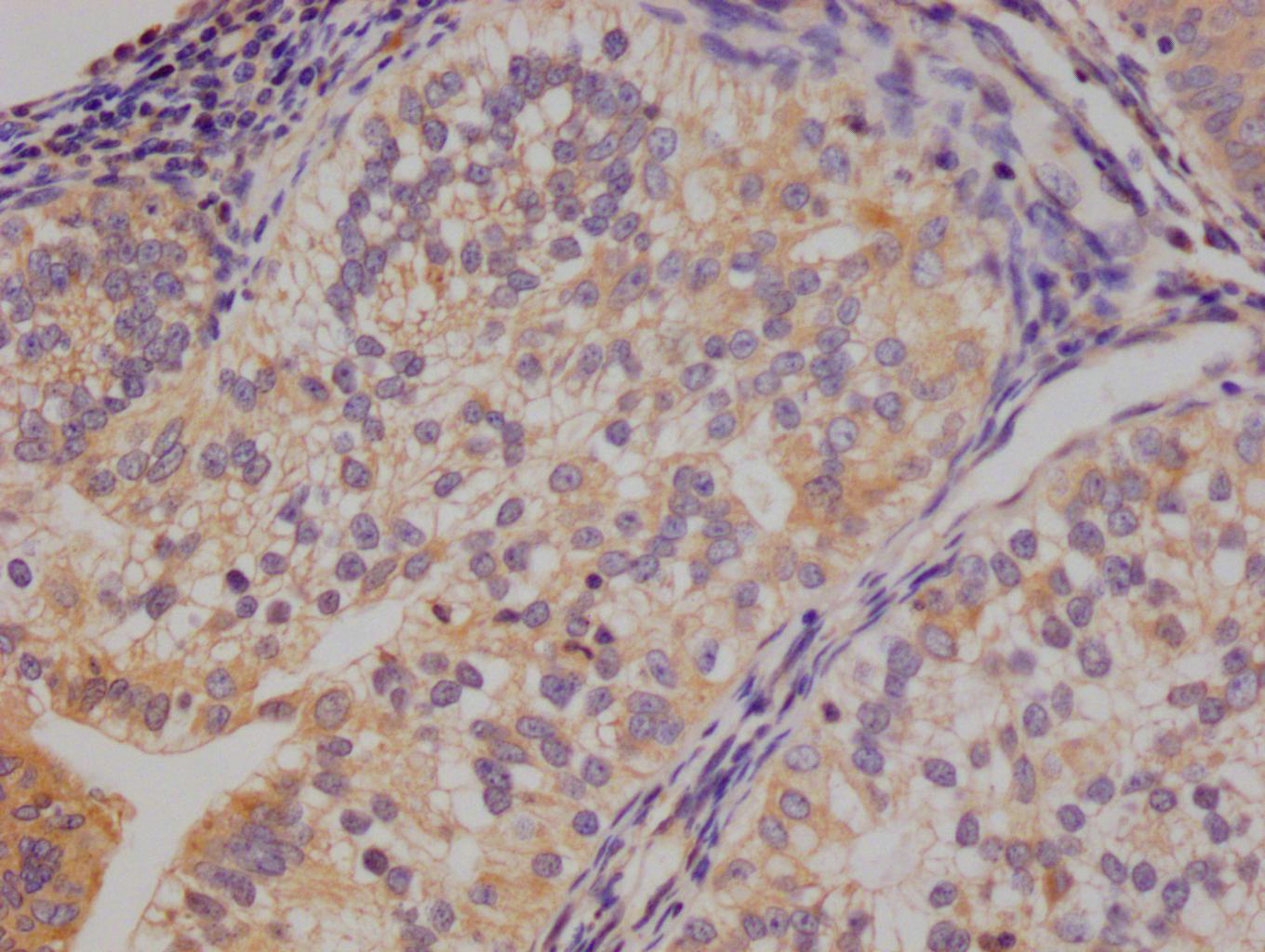
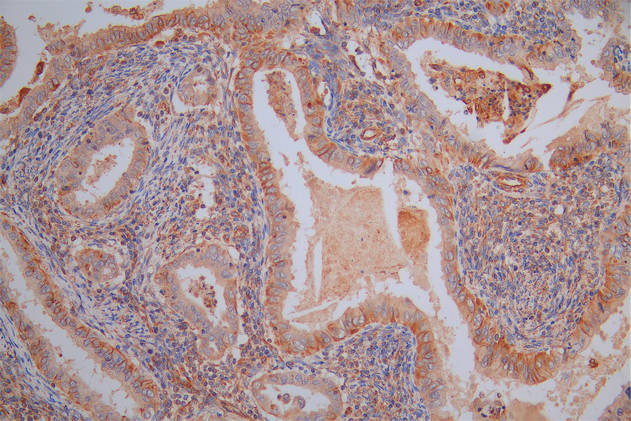
The image on the left is immunohistochemistry of paraffin-embedded Human breast cancer tissue using CSB-PA697715(TRAIP Antibody) at dilution 1/40, on the right is treated with fusion protein. (Original magnification: ×200)
-
-
其他:
产品详情
-
Uniprot No.:Q9BWF2
-
基因名:TRAIP
-
别名:RING finger protein 206 antibody; RNF206 antibody; TRAF Interacting Protein antibody; TRAF-interacting protein antibody; Traip antibody; TRAIP_HUMAN antibody; TRIP antibody
-
宿主:Rabbit
-
反应种属:Human
-
免疫原:Fusion protein of Human TRAIP
-
免疫原种属:Homo sapiens (Human)
-
标记方式:Non-conjugated
-
抗体亚型:IgG
-
纯化方式:Antigen affinity purification
-
浓度:It differs from different batches. Please contact us to confirm it.
-
保存缓冲液:-20°C, pH7.4 PBS, 0.05% NaN3, 40% Glycerol
-
产品提供形式:Liquid
-
应用范围:ELISA,IHC
-
推荐稀释比:
Application Recommended Dilution ELISA 1:2000-1:10000 IHC 1:30-1:150 -
Protocols:
-
储存条件:Upon receipt, store at -20°C or -80°C. Avoid repeated freeze.
-
货期:Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time maybe differs from different purchasing way or location, please kindly consult your local distributors for specific delivery time.
相关产品
靶点详情
-
功能:E3 ubiquitin ligase required to protect genome stability in response to replication stress. Acts as a key regulator of interstrand cross-link repair, which takes place when both strands of duplex DNA are covalently tethered together, thereby blocking replication and transcription. Controls the choice between the two pathways of replication-coupled interstrand-cross-link repair by mediating ubiquitination of MCM7 subunit of the CMG helicase complex. Short ubiquitin chains on MCM7 promote recruitment of DNA glycosylase NEIL3. If the interstrand cross-link cannot be cleaved by NEIL3, the ubiquitin chains continue to grow on MCM7, promoting the unloading of the CMG helicase complex by the VCP/p97 ATPase, enabling the Fanconi anemia DNA repair pathway. Only catalyzes ubiquitination of MCM7 when forks converge. Also involved in the repair of covalent DNA-protein cross-links (DPCs) during DNA synthesis: promotes ubiquitination of DPCs, leading to their degradation by the proteasome. Has also been proposed to play a role in promoting translesion synthesis by mediating the assembly of 'Lys-63'-linked poly-ubiquitin chains on the Y-family polymerase POLN in order to facilitate bypass of DNA lesions and preserve genomic integrity. The function in translesion synthesis is however controversial. Acts as a regulator of the spindle assembly checkpoint. Also acts as a negative regulator of innate immune signaling by inhibiting activation of NF-kappa-B mediated by TNF. Negatively regulates TLR3/4- and RIG-I-mediated IRF3 activation and subsequent IFNB1 production and cellular antiviral response by promoting 'Lys-48'-linked polyubiquitination of TNK1 leading to its proteasomal degradation.
-
基因功能参考文献:
- The TRAIP coiled-coil domain altered its stoichiometry between dimer and trimer in a concentration-dependent manner. Additionally, the TRAIP RING domain induced even higher-ordered assembly, which was necessary for interacting with the TRAF-N domain of TRAF2 but not TRAF1. PMID: 30127245
- TRIP interacts with transforming growth factor beta-activated kinase 1 (TAK1) and promotes K48-linked polyubiquitination of TAK1 in rheumatoid arthritis fibroblast-Like synoviocytes PMID: 27847407
- Taken together, these findings improve the understanding clinical implication of TRAIP in various diseases including primordial dwarfism and cancers. PMID: 26820530
- cell cycle-dependent transcription of the TRAIP gene by E2F1, E2F2, and E2F4 and rapid protein degradation leads to cell cycle-dependent expression with a maximum in G2/M PMID: 26369285
- TRAIP/RNF206 is required for recruitment of RAP80 to sites of DNA damage.( PMID: 26781088
- These findings establish TRAIP as a PCNA-binding ubiquitin ligase with an important role in protecting genome integrity after obstacles to DNA replication. PMID: 26711499
- TRAIP is a component of the DNA damage response to replication-blocking DNA lesions.TRAIP promotes DNA damage response during genome replication and is mutated in primordial dwarfism. PMID: 26595769
- The TRAIP ubiquitin ligase activity is functionally required for the spindle assembly checkpoint control. PMID: 25335891
- a number of TRAIP mutants were used to define the TRAIP molecular domains responsible for its homo-dimerization. A co-immunoprecipitation assay indicated that the TRAIP forms homo-dimerization through the CC domain PMID: 26093298
- Data indicate that TRAF interacting protein TRIP negatively regulates the TNFR-associated factor 2 (TRAF2) ubiquitin-dependent pathway by modulating the TRAF2-sphingosine 1-phosphate (S1P) interaction. PMID: 25716317
- observed enhanced ubiquitylation of Poleta by TRIP E3 ligase and show that TRIP promotes Poleta localization to nuclear foci PMID: 24553286
- The TRAF-interacting protein (TRIP) is a regulator of keratinocyte proliferation. PMID: 21068752
- CYLD interacts with TRIP and regulates negatively nuclear factor kappaB activation by tumor necrosis factor. PMID: 14676304
- The overexpression of TRIP sensitizes cells to TNF-induced apoptosis, an effect that can be reversed by the coexpression of Syk. PMID: 19151749
显示更多
收起更多
-
相关疾病:Seckel syndrome 9 (SCKL9)
-
亚细胞定位:Nucleus, nucleoplasm. Nucleus, nucleolus. Chromosome. Cytoplasm. Cytoplasm, perinuclear region.
-
数据库链接:
HGNC: 30764
OMIM: 605958
KEGG: hsa:10293
STRING: 9606.ENSP00000328203
UniGene: Hs.517972
Most popular with customers
-
-
YWHAB Recombinant Monoclonal Antibody
Applications: ELISA, WB, IF, FC
Species Reactivity: Human, Mouse, Rat
-
Phospho-YAP1 (S127) Recombinant Monoclonal Antibody
Applications: ELISA, WB, IHC
Species Reactivity: Human
-
-
-
-
-