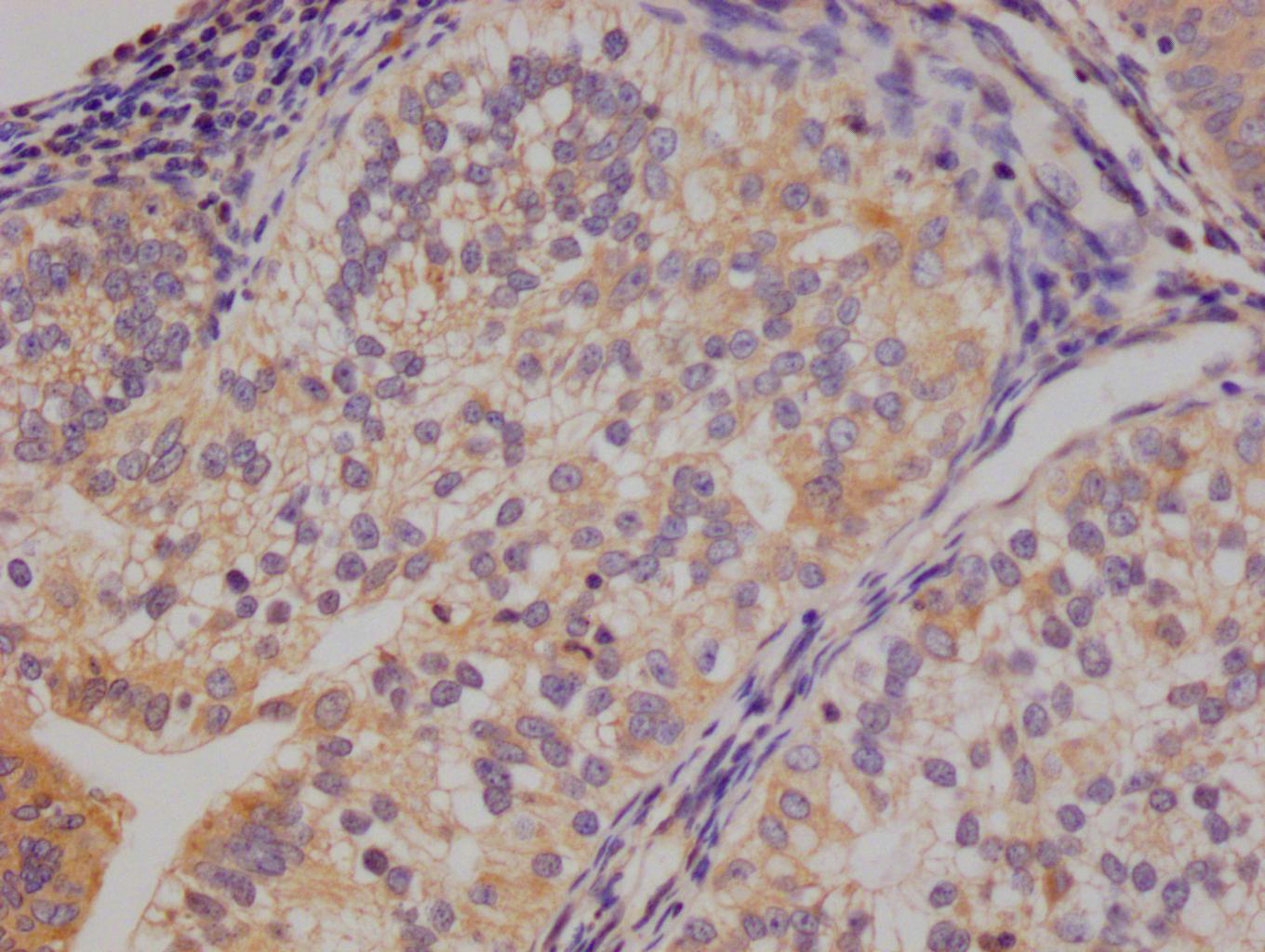
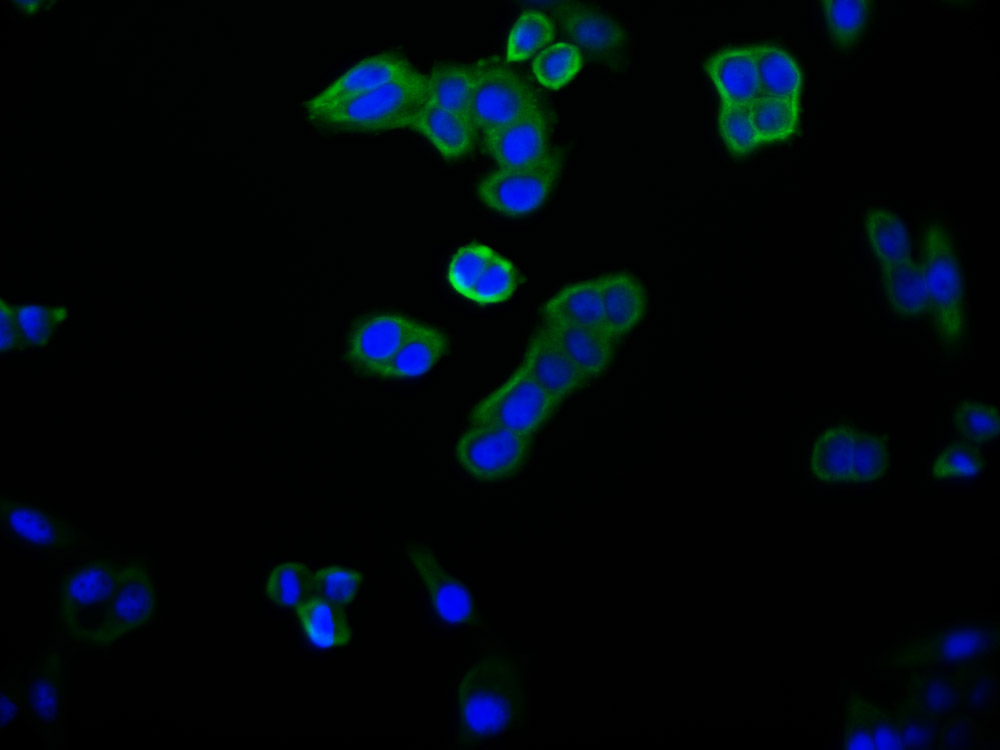
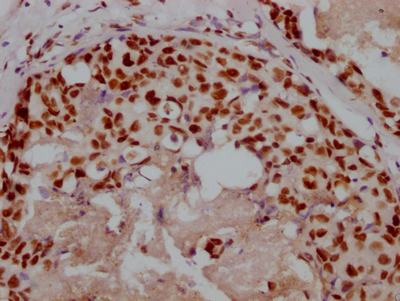
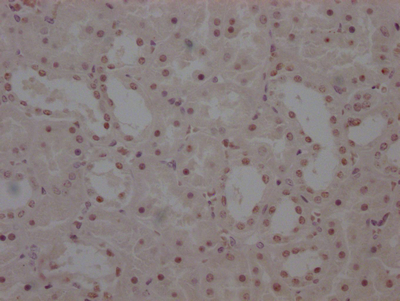
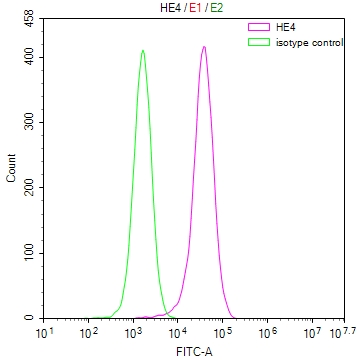
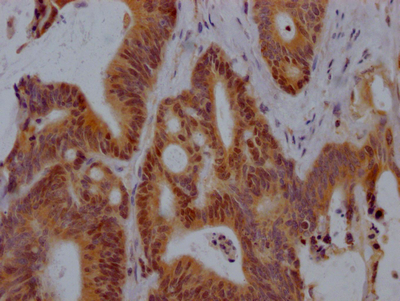
TPP1 Antibody
-
货号:CSB-PA024113GA01HU
-
规格:¥3,900
-
其他:
产品详情
-
Uniprot No.:O14773
-
基因名:
-
别名:Cell growth inhibiting gene 1 protein antibody; Cell growth-inhibiting gene 1 protein antibody; Ceroid lipofuscinosis neuronal 2 antibody; Ceroid lipofuscinosis neuronal 2 late infantile (Jansky Bielschowsky disease) antibody; Ceroid lipofuscinosis neuronal 2 late infantile antibody; CLN 2 antibody; CLN2 antibody; GIG 1 antibody; GIG1 antibody; Growth inhibiting protein 1 antibody; LPIC antibody; Lysosomal pepstatin insensitive protease antibody; Lysosomal pepstatin-insensitive protease antibody; MGC21297 antibody; TPP 1 antibody; TPP I antibody; TPP-1 antibody; TPP-I antibody; Tpp1 antibody; TPP1_HUMAN antibody; TPPI antibody; Tripeptidyl aminopeptidase antibody; Tripeptidyl peptidase I antibody; Tripeptidyl-peptidase 1 antibody; Tripeptidyl-peptidase I antibody
-
宿主:Rabbit
-
反应种属:Human,Mouse,Rat
-
免疫原:Human TPP1
-
免疫原种属:Homo sapiens (Human)
-
抗体亚型:IgG
-
纯化方式:Antigen Affinity Purified
-
浓度:It differs from different batches. Please contact us to confirm it.
-
保存缓冲液:PBS with 0.1% Sodium Azide, 50% Glycerol, pH 7.3. -20°C, Avoid freeze / thaw cycles.
-
产品提供形式:Liquid
-
应用范围:ELISA,WB,IHC
-
Protocols:
-
储存条件:Upon receipt, store at -20°C or -80°C. Avoid repeated freeze.
-
货期:Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time maybe differs from different purchasing way or location, please kindly consult your local distributors for specific delivery time.
相关产品
靶点详情
-
功能:Lysosomal serine protease with tripeptidyl-peptidase I activity. May act as a non-specific lysosomal peptidase which generates tripeptides from the breakdown products produced by lysosomal proteinases. Requires substrates with an unsubstituted N-terminus.
-
基因功能参考文献:
- The reports the crystal structure of the N-terminal domain of TIN2 in complex with TIN2-binding motifs from TPP1 and TRF2, revealing how TIN2 interacts cooperatively with TPP1 and TRF2. PMID: 29160297
- TPP1 cleaves and destabilizes fibrillar amyloid-beta at multiple sites in a time- and pH-dependent manner. PMID: 29378960
- To confirm clinical suspicion of CLN2 disease, the recommended gold standard for laboratory diagnosis is demonstration of deficient TPP1 enzyme activity (in leukocytes, fibroblasts, or dried blood spots) and the identification of causative mutations in each allele of the TPP1/CLN2 gene. PMID: 27553878
- These studies indicate that optimal treatment outcomes for CLN2 disease may require delivery of TPP1 systemically as well as directly to the central nervous system. PMID: 28079862
- TPP1 is overexpressed in hepatocellular carcinoma tissues and significantly correlated with poor prognosis of hepatocellular carcinoma patients.RFX5 acts as a direct positive transcriptional regulator of TPP1 in hepatocellular carcinoma. PMID: 27840983
- TPP1(CLN2) mutation is associated with neuronal ceroid lipofuscinosis. PMID: 24271013
- To our knowledge, our results bring the first evidence of a mechanism that links TPP-1 deficiency and oxidative stress-induced changes in mitochondrial morphology. PMID: 23249249
- hypothesize that loss of function variants abolishing TPP1 enzyme activity lead to CLN2 disease, whereas variants that diminish TPP1 enzyme activity lead to SCAR7 PMID: 23418007
- This study demonistrated that the CLN2 gene 4 mutation in late infantile neuronal ceroid lipofuscinosis. PMID: 22832778
- TPP1 mutants utilize the advantages of a zebrafish model for understanding the pathogenesis of late infantile (or classic late infantile neuronal ceroid lipofuscinosis) disease. PMID: 23587805
- The variant juvenile phenotype comprises approximately 50% of CLN2 in South America. The five most frequent South American mutations comprise 66% of pathological alleles. PMID: 23266810
- Gemfibrozil and fenofibrate, Food and Drug Administration-approved lipid-lowering drugs, up-regulate tripeptidyl-peptidase 1 in brain cells via peroxisome proliferator-activated receptor alpha and may have implications in late infantile Batten disease therapy PMID: 22989886
- Studies indicate that TPP-I is the only member of the sedolisin family that has been shown to exhibit tripeptidyl peptidase activity and is related to the fatal hereditary disease, Batten disease. PMID: 22016395
- Intrathecal human tripeptidyl-peptidase 1 administration reduces lysosomal storage in a canine model of late infantile neuronal ceroid lipofuscinosis. PMID: 21784683
- the critical residues in the TPPI catalysis and its structure-function analysis PMID: 20689811
- Data show that most TPPI variants displayed obstructed transport to the lysosomes. PMID: 20340139
- The s conducted a phase I study of late infantile neuronal ceroid lipofuscinosis using an adenoassociated virus serotype 2 (AAV2) vector containing the deficient CLN2 gene (AAV2(CU)hCLN2). PMID: 20672930
- The clinical, biochemical, and molecular genetic aspects of lysosomal storage disorders are discussed in this review PMID: 12125808
- Data show that three neuronal ceroid lipofuscinoses disease forms with similar tissue pathology are connected at the molecular level: CLN5 polypeptides directly interact with the CLN2 and CLN3 proteins PMID: 12134079
- Missense mutations, R127Q, N286S, and T353P represent novel, previously not described alleles. PMID: 12376936
- human tripeptidyl-peptidase I is processed by a serine protease to the mature, active form in vivo PMID: 12488460
- a novel mutation in neuronal ceroid lipofuscinosis PMID: 12698559
- CLN2 gene mutations may result in low cerebrospinal fluid pterin production in classical neuronal ceroid lipofuscinoses of late infantile onset. PMID: 12950156
- human tripeptidyl-peptidase I must be N-glycosylated for folding, trafficking, and stability PMID: 14702339
- mutant Asn286Ser CLN2 lacks one oligosaccharide chain resulting in enzymatic inactivation PMID: 14736728
- intramolecular (unimolecular) mechanism of TPP I activation and autoprocessing PMID: 15143070
- TPP-I is the predominant proteolytic enzyme responsible for the intracellular degradation of neuromedin B PMID: 15158442
- Functional analyses of CLN2 mutations reveal transport disruption of tripeptidyl-peptidase I to lysosomes. PMID: 15317752
- tripeptidyl-peptidase I activation, activity, and stability are regulated by glycosaminoglycans PMID: 15582991
- Ser475 and Asp360, also Glu272, Asp276, and Asp327 are important for catalytic activity of tripeptidyl peptidase I PMID: 15733845
- Substrate-binding cleft of TPP-I composed of only 6 subsites; TPP-I prefers bulky and hydrophobic amino acid residues at P(1) position and Ala, Arg, or Asp at P(2) position; hydrophilic interactions at the S(2) subsite are necessary for TPP-I. PMID: 16091586
- Mutational screening of CLCN2 gene, revealed a homozygous mutation G2003C (exon 17), leading to a Ser/Thr substitution at the codon 668, in two of the three in malignant migrating partial seizures patients. PMID: 16168594
- there is a close correlation between CLN2 and CLN1 expression and colorectal carcinoma progression and metastasis and suggest that they may be potential molecular targets PMID: 16518810
- Clinical features, histological findings, and genetic study reveal that CLN2 type is the most common form of neuronal ceroid lipofuscinosis. There is male predominance of 90.1% in this part of the Arab world. PMID: 17690061
- CLN2/TPP1 deficiency: the novel mutation IVS7-10A>G causes intron retention and is associated with a mild disease phenotype. PMID: 17959406
- the tripeptidyl peptidase I prosegment is a potent, slow-binding inhibitor of its cognate enzyme PMID: 18411270
- Lysosome-related genes, such as CLN2, CLN3, and HEXB, may be involved in the pathogenesis of adipose tissue hypertrophy in TED. PMID: 18552385
- Structure of tripeptidyl-peptidase I provides insight into the molecular basis of late infantile neuronal ceroid lipofuscinosis. PMID: 19038966
- Crystal structure and autoactivation pathway of the precursor form of human tripeptidyl-peptidase 1, the enzyme deficient in late infantile ceroid lipofuscinosis PMID: 19038967
- This novel deletion mutation in the CLN2 gene in a family of Arab origin from Israel sheds further light on the epidemiology of neuronal ceroid lipofuscinosis as a worldwide disease PMID: 19748052
- functional analysis of variants expressed in CHO cells PMID: 11462245
显示更多
收起更多
-
相关疾病:Ceroid lipofuscinosis, neuronal, 2 (CLN2); Spinocerebellar ataxia, autosomal recessive, 7 (SCAR7)
-
亚细胞定位:Lysosome. Melanosome.
-
组织特异性:Detected in all tissues examined with highest levels in heart and placenta and relatively similar levels in other tissues.
-
数据库链接:
HGNC: 2073
OMIM: 204500
KEGG: hsa:1200
STRING: 9606.ENSP00000299427
UniGene: Hs.523454
Most popular with customers
-
-
YWHAB Recombinant Monoclonal Antibody
Applications: ELISA, WB, IF, FC
Species Reactivity: Human, Mouse, Rat
-
Phospho-YAP1 (S127) Recombinant Monoclonal Antibody
Applications: ELISA, WB, IHC
Species Reactivity: Human
-
-
-
-
-