-
中文名称:牛铜锌-过氧化物岐化酶(Cu/Zn-SOD)酶联免疫试剂盒
-
货号:CSB-E14090B
-
规格:96T/48T
-
价格:¥4200/¥3000
-
其他:
产品详情
-
产品描述:
This Bovine SOD1 ELISA Kit was designed for the quantitative measurement of Bovine SOD1 protein in serum, plasma. It is a Sandwich ELISA kit, its detection range is 15.6 ng/mL-1000 ng/mL and the sensitivity is 3.9 ng/mL.
-
别名:SOD1 ELISA Kit; Superoxide dismutase [Cu-Zn] ELISA Kit; EC 1.15.1.1 ELISA Kit
-
缩写:
-
Uniprot No.:
-
种属:Bos taurus (Bovine)
-
样本类型:serum, plasma
-
检测范围:15.6 ng/mL-1000 ng/mL
-
灵敏度:3.9 ng/mL
-
反应时间:1-5h
-
样本体积:50-100ul
-
检测波长:450 nm
-
研究领域:Metabolism
-
测定原理:quantitative
-
测定方法:Sandwich
-
精密度:
Intra-assay Precision (Precision within an assay): CV%<8% Three samples of known concentration were tested twenty times on one plate to assess. Inter-assay Precision (Precision between assays): CV%<10% Three samples of known concentration were tested in twenty assays to assess. -
线性度:
To assess the linearity of the assay, samples were spiked with high concentrations of bovine Cu/Zn-SOD in various matrices and diluted with the Sample Diluent to produce samples with values within the dynamic range of the assay. Sample Serum(n=4) 1:1 Average % 97 Range % 92-104 1:2 Average % 91 Range % 85-97 1:4 Average % 90 Range % 88-95 1:8 Average % 100 Range % 96-105 -
回收率:
The recovery of bovine Cu/Zn-SOD spiked to levels throughout the range of the assay in various matrices was evaluated. Samples were diluted prior to assay as directed in the Sample Preparation section. Sample Type Average % Recovery Range Serum (n=5) 90 87-94 EDTA plasma (n=4) 101 103-105 -
标准曲线:
These standard curves are provided for demonstration only. A standard curve should be generated for each set of samples assayed. 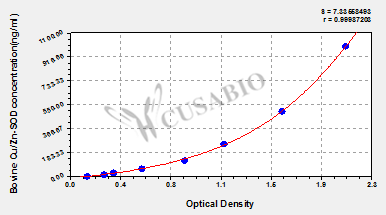
ng/ml OD1 OD2 Average Corrected 1000 2.014 2.115 2.065 1.929 500 1.537 1.640 1.589 1.453 250 1.201 1.108 1.155 1.019 125 0.878 0.844 0.861 0.725 62.5 0.541 0.555 0.548 0.412 31.2 0.342 0.329 0.336 0.200 15.6 0.251 0.272 0.262 0.126 0 0.137 0.135 0.136 -
数据处理:
-
货期:3-5 working days
引用文献
- Growth performance, biochemical parameters and antioxidant status of heat-stressed calves fed on a diet containing Moringa oleifera leaves powder E Wer,/,2024
- Invitro Effect of Zinc Treatment on the Antioxidant Status of Heat Stressed Peripheral Blood Mononuclear Cells of Periparturient Sahiwal and Karan Fries Cows - A Comparative Study Sheikh A A.et al,Journal of Animal Research,2015
- Invitro Effect of Zinc Treatment on the Antioxidant Status of Heat Stressed Peripheral Blood Mononuclear Cells of Periparturient Sahiwal and Karan Fries Cows- A Comparative Study Aasif Ahmad Sheikh. et al,Journal of Animal Research,2015
相关产品
靶点详情
-
功能:Destroys radicals which are normally produced within the cells and which are toxic to biological systems.
-
基因功能参考文献:
- Results indicate that variants of the PRLH and SOD1 genes are associated with heat tolerance in Chinese cattle. PMID: 30079537
- The three-dimensional structure of bSOD1 reveals the imidazole ring of His19 localized within 5A from the alpha-carbon of Gly31 providing a structural basis that copper ion, most likely coordinated by His19, catalyzes the specific cleavage reaction PMID: 26872685
- SOD catalyzes reversal of autoxidation manifesting as its inhibition. SOD saves catechols from autoxidation and extends their bioavailability PMID: 25416864
- antioxidative enzymatic mechanisms in bovine placental tissues are represented by superoxide dismutase 1 and glutathione peroxidase, which show the changes in their expression during improper placental release PMID: 23398331
- Results sugget thet Copper/Zinc superoxide dismutase (SOD1) may play a role in controlling intraluteal prostaglandin F2alph and reactive oxygen species action during functional and structural luteolysis. PMID: 23101731
- ALOX5AP, CPNE3, IL1R2, IL6, TLR2, TLR4, and THY1 were upregulated in blood polymorphonuclear cells in negative energy balance versus positive energy balance cows. PMID: 20072847
- Acute elevation of SOD may represent a response of luteal endothelial cells to protect themselves against oxidative stress induced by PGF during functional luteolysis. PMID: 20519832
- At room temperature (25.0 degrees C) and higher, the addition of high concentrations of polymer is found to significantly enhance the affinity of SOD for catalase. PMID: 20682270
- Capillary electrophoresis and mass spectrometry to study the different structures of bovine SOD-1. In both cases, an average molecular mass corresponding to the apo-monomer SOD-1 was calculated. PMID: 20411580
- flexibility of the metal sites involved in present a single-crystal X-ray diffraction study of Cu,Zn superoxide dismutase in space group P212121 at 0.57 GPa. The crystal structure (hpSOD) was determined and refined at 2 A degrees resolution. PMID: 20516618
- expression profile in follicles: oocytes (SOD1 throughout ooplasm & nucleoplasm); cumulus cells (no SOD1 detected); granulosa cells (expressed SOD1); follicular fluid (small follicles show increased amounts of SOD1 in comparison with large follicles) PMID: 20197373
- Bovine erythrocyte Cu,Zn-superoxide dismutase (BESOD) is a dimeric enzyme composed of identical subunits associated through unusually strong non-covalent interactions. PMID: 14688234
- Raman spectrum analysis strongly suggests that the His41-mediated hydrogen bond bridge of Cu-Zn superoxide dismutase plays a crucial role in keeping the protein structure suitable for highly efficient catalytic reactions. PMID: 15096035
- HCO(3)(-)-derived oxidant does not alter significantly the Cu(II) active site geometry and histidine coordination to Cu(II) in SOD1 as does H(2)O(2) alone PMID: 15123612
- copper- and carbonate radical anion-mediated oxidations have roles in hydrogen peroxide-induced Cu,Zn-superoxide dismutase-centered radical formation PMID: 15607903
- SOD1 mutants gain fatty acid binding abilities based on their structural instability and form cytotoxic granular aggregates PMID: 15799963
- The kinetics of thermal dissociation of superoxide dismutase (SOD) was studied in 0.05 M Tris-HCl buffer at pH 7.4 containing 10(-4) M EDTA. PMID: 16202231
- Cu,Zn-superoxide dismutase (CuZnSOD) catalyzes the reductive decomposition of S-nitroso-L-glutathione (GSNO) in the presence of thiols such as L-glutathione (GSH). PMID: 17042490
- communication between the two monomers of SOD1 such that the binding of one zinc ion per homodimer has a more profound effect on the homodimeric protein structure than the binding of subsequent metal ions PMID: 17381088
- DNA accelerates the formation of SOD1 aggregates and is incorporated into SOD1 aggregates. SOD1 association with DNA, driven by electrostatic interactions, can restrict the orientation of SOD1 molecules and increase a SOD1 population along DNA strands. PMID: 17469801
- The results suggest that under cellular conditions ( approximately 5 mM bicarbonate) zinc-deficient SOD1 peroxidation could play a pathogenic role in neurodegenerative diseases. PMID: 17729118
- The expression of SOD1 and SOD2 through the course of the estrous cycle is reported. PMID: 18572235
- diminished hepatic protein nitration in the SOD1-/- mice was not directly related to plasma nitrite and nitrate concentrations PMID: 18573333
- DNAs tested are simultaneously condensed into a nanoparticle with a specific morphology during SOD1 aggregation, revealing that SOD1 aggregation and DNA condensation are two concurrent phenomena. PMID: 18690666
- Peroxymonocarbonate (HOOCO(2)(-)) is a key intermediate in the SOD1 peroxidase cycle and identify this species as the precursor of carbonate radical anions. PMID: 19286663
显示更多
收起更多
-
亚细胞定位:Cytoplasm. Nucleus.
-
蛋白家族:Cu-Zn superoxide dismutase family
-
数据库链接:
Most popular with customers
-
Human Transforming Growth factor β1,TGF-β1 ELISA kit
Detect Range: 23.5 pg/ml-1500 pg/ml
Sensitivity: 5.8 pg/ml
-
-
-
Mouse Tumor necrosis factor α,TNF-α ELISA Kit
Detect Range: 7.8 pg/ml-500 pg/ml
Sensitivity: 1.95 pg/ml
-
-
-
-













