Recombinant Sleeping disease virus Structural polyprotein
-
中文名称:Recombinant Sleeping disease virus Structural polyprotein
-
货号:CSB-CF823134SJAE
-
规格:
-
来源:in vitro E.coli expression system
-
其他:
产品详情
-
基因名:N/A
-
Uniprot No.:
-
别名:Structural polyprotein; p130
-
种属:Sleeping disease virus (SDV)
-
蛋白长度:Full Length of Mature Protein
-
表达区域:861-1322
-
氨基酸序列YEHTVVVPMDPRAPSYEAVINRNGYDPLKLTIAVNFTVISPTTALEYWTCAGVPVVEPPH VGCCTSVSCPSDLSTLHAFTGKAVSDVHCDVHTNVYPLLWGAAHCFCSTENTQVSAVAAT VSEFCAQDSERAEAFSVHSSSVTAEILVTLGEVVTAVHVYVDGVTSARGTDLKIVAGPIT TDYSPFDRKVVRIGEEVYNYDWPPYGAGRPGTFGDIQARSTNYVKPNDLYGDIGIEVLQP TNDHVHVAYTYTTSGLLRWLQDAPKPLSVTAPHGCKISANPLLALDCGVGAVPMSINIPD AKFTRKLKDPKPSALKCVVDSCEYGVDYGGAATITYEGHEAGKCGIHSLTPGVPLRTSVV EVVAGANTVKTTFSSPTPEVTLEVEICSAIVKCASECTPPKEHVVAARPRHGSDTGGYIS GPAMRWAGRIVGNPSGPVSSSLAVTYCVVKKCRSKRIRIVKS
Note: The complete sequence including tag sequence, target protein sequence and linker sequence could be provided upon request. -
蛋白标签:N-terminal 10xHis-tagged
-
产品提供形式:Liquid or Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
缓冲液:Lyophilized from Tris/PBS-based buffer, 6% Trehalose, pH 8.0
-
储存条件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保质期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
货期:Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事项:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet & COA:Please contact us to get it.
相关产品
靶点详情
-
功能:Forms an icosahedral capsid with a T=4 symmetry composed of 240 copies of the capsid protein surrounded by a lipid membrane through which penetrate 80 spikes composed of trimers of E1-E2 heterodimers. The capsid protein binds to the viral RNA genome at a site adjacent to a ribosome binding site for viral genome translation following genome release. Possesses a protease activity that results in its autocatalytic cleavage from the nascent structural protein. Following its self-cleavage, the capsid protein transiently associates with ribosomes, and within several minutes the protein binds to viral RNA and rapidly assembles into icosahedric core particles. The resulting nucleocapsid eventually associates with the cytoplasmic domain of the spike glycoprotein E2 at the cell membrane, leading to budding and formation of mature virions. In case of infection, new virions attach to target cells and after clathrin-mediated endocytosis their membrane fuses with the host endosomal membrane. This leads to the release of the nucleocapsid into the cytoplasm, followed by an uncoating event necessary for the genomic RNA to become accessible. The uncoating might be triggered by the interaction of capsid proteins with ribosomes. Binding of ribosomes would release the genomic RNA since the same region is genomic RNA-binding and ribosome-binding. Specifically inhibits interleukin-1 receptor-associated kinase 1/IRAK1-dependent signaling during viral entry, representing a means by which the alphaviruses may evade innate immune detection and activation prior to viral gene expression.; Provides the signal sequence for the translocation of the precursor of protein E3/E2 to the host endoplasmic reticulum. Furin-cleaved E3 remains associated with spike glycoprotein E1 and mediates pH protection of the latter during the transport via the secretory pathway. After virion release from the host cell, the assembly protein E3 is gradually released in the extracellular space.; Plays a role in viral attachment to target host cell, by binding to the cell receptor. Synthesized as a p62 precursor which is processed by furin at the cell membrane just before virion budding, giving rise to E2-E1 heterodimer. The p62-E1 heterodimer is stable, whereas E2-E1 is unstable and dissociate at low pH. p62 is processed at the last step, presumably to avoid E1 fusion activation before its final export to cell surface. E2 C-terminus contains a transitory transmembrane that would be disrupted by palmitoylation, resulting in reorientation of the C-terminal tail from lumenal to cytoplasmic side. This step is critical since E2 C-terminus is involved in budding by interacting with capsid proteins. This release of E2 C-terminus in cytoplasm occurs lately in protein export, and precludes premature assembly of particles at the endoplasmic reticulum membrane.; Constitutive membrane protein involved in virus glycoprotein processing, cell permeabilization, and the budding of viral particles. Disrupts the calcium homeostasis of the cell, probably at the endoplasmic reticulum level. This leads to cytoplasmic calcium elevation. Because of its lipophilic properties, the 6K protein is postulated to influence the selection of lipids that interact with the transmembrane domains of the glycoproteins, which, in turn, affects the deformability of the bilayer required for the extreme curvature that occurs as budding proceeds. Present in low amount in virions, about 3% compared to viral glycoproteins.; Class II viral fusion protein. Fusion activity is inactive as long as E1 is bound to E2 in mature virion. After virus attachment to target cell and endocytosis, acidification of the endosome would induce dissociation of E1/E2 heterodimer and concomitant trimerization of the E1 subunits. This E1 trimer is fusion active, and promotes release of viral nucleocapsid in cytoplasm after endosome and viral membrane fusion. Efficient fusion requires the presence of cholesterol and sphingolipid in the target membrane. Fusion is optimal at levels of about 1 molecule of cholesterol per 2 molecules of phospholipids, and is specific for sterols containing a 3-beta-hydroxyl group.
-
亚细胞定位:[Capsid protein]: Virion. Host cytoplasm. Host cell membrane. Host nucleus.; [Spike glycoprotein E2]: Virion membrane; Single-pass type I membrane protein. Host cell membrane; Single-pass type I membrane protein.; [6K protein]: Host cell membrane; Multi-pass membrane protein. Virion membrane; Multi-pass membrane protein.; [Spike glycoprotein E1]: Virion membrane; Single-pass type I membrane protein. Host cell membrane; Single-pass type I membrane protein.
-
数据库链接:
KEGG: vg:1729818
Most popular with customers
-
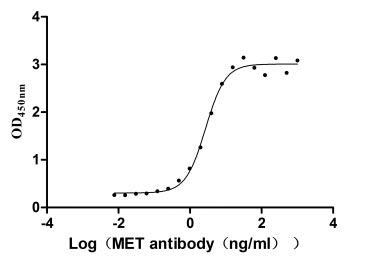
Recombinant Human Hepatocyte growth factor receptor (MET), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Lymphotoxin-alpha (LTA) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
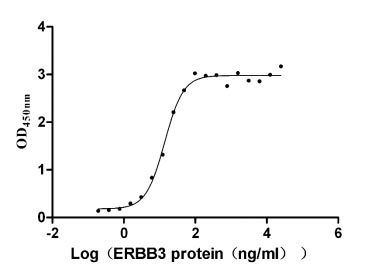
Recombinant Human Receptor tyrosine-protein kinase erbB-3 (ERBB3), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Heat-stable enterotoxin receptor (GUCY2C), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Complement component C1q receptor (CD93), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Mouse Complement component C1q receptor (Cd93), partial (Active)
Express system: Mammalian cell
Species: Mus musculus (Mouse)
-
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
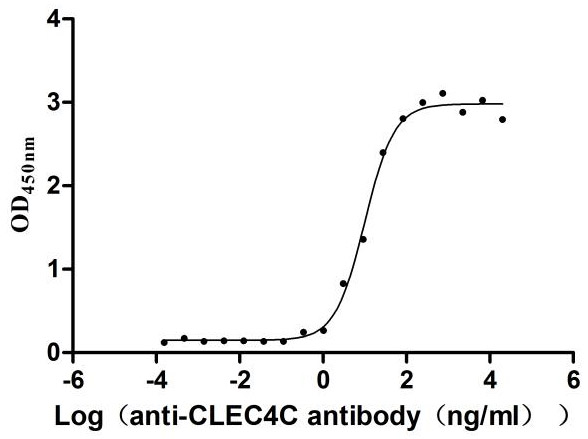
Recombinant Human C-type lectin domain family 4 member C (CLEC4C), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)