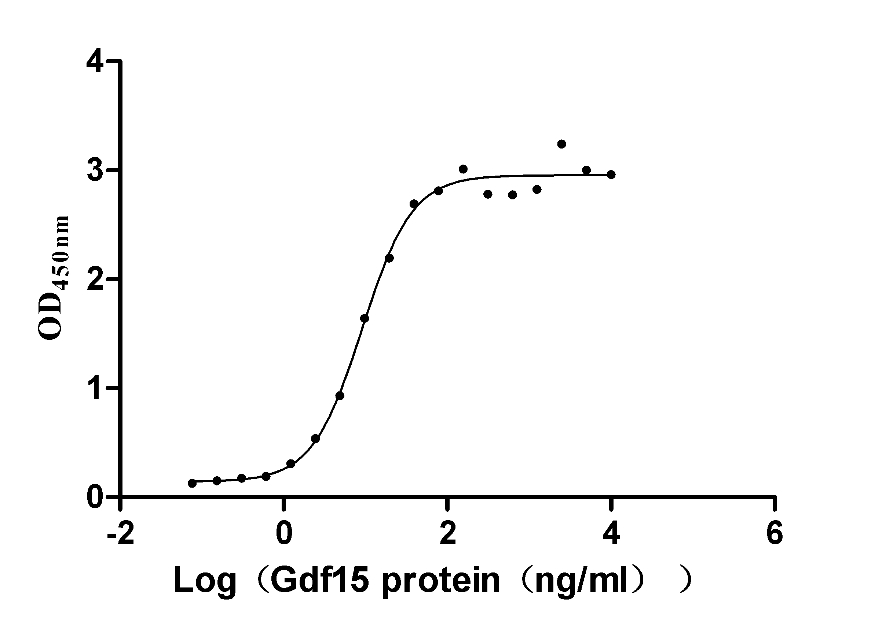
Recombinant Mouse Potassium voltage-gated channel subfamily A member 1 (Kcna1)
-
中文名称:小鼠Kcna1重组蛋白
-
货号:CSB-CF012005MO
-
规格:
-
来源:in vitro E.coli expression system
-
其他:
产品详情
-
基因名:Kcna1
-
Uniprot No.:
-
别名:Kcna1; Potassium voltage-gated channel subfamily A member 1; MBK1; MKI; Voltage-gated potassium channel subunit Kv1.1
-
种属:Mus musculus (Mouse)
-
蛋白长度:Full length protein
-
表达区域:1-495
-
氨基酸序列MTVMSGENADEASTAPGHPQDGSYPRQADHDDHECCERVVINISGLRFETQLKTLAQFPN TLLGNPKKRMRYFDPLRNEYFFDRNRPSFDAILYYYQSGGRLRRPVNVPLDMFSEEIKFY ELGEEAMEKFREDEGFIKEEERPLPEKEYQRQVWLLFEYPESSGPARVIAIVSVMVILIS IVIFCLETLPELKDDKDFTGTIHRIDNTTVIYTSNIFTDPFFIVETLCIIWFSFELVVRF FACPSKTDFFKNIMNFIDIVAIIPYFITLGTEIAEQEGNQKGEQATSLAILRVIRLVRVF RIFKLSRHSKGLQILGQTLKASMRELGLLIFFLFIGVILFSSAVYFAEAEEAESHFSSIP DAFWWAVVSMTTVGYGDMYPVTIGGKIVGSLCAIAGVLTIALPVPVIVSNFNYFYHRETE GEEQAQLLHVSSPNLASDSDLSRRSSSTISKSEYMEIEEDMNNSIAHYRQANIRTGNCTT ADQNCVNKSKLLTDV
Note: The complete sequence including tag sequence, target protein sequence and linker sequence could be provided upon request. -
蛋白标签:N-terminal 10xHis-tagged
-
产品提供形式:Liquid or Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
缓冲液:Lyophilized from Tris/PBS-based buffer, 6% Trehalose, pH 8.0
-
储存条件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保质期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
货期:Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事项:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet & COA:Please contact us to get it.
相关产品
靶点详情
-
功能:Voltage-gated potassium channel that mediates transmembrane potassium transport in excitable membranes, primarily in the brain and the central nervous system, but also in the kidney. Contributes to the regulation of the membrane potential and nerve signaling, and prevents neuronal hyperexcitability. Forms tetrameric potassium-selective channels through which potassium ions pass in accordance with their electrochemical gradient. The channel alternates between opened and closed conformations in response to the voltage difference across the membrane. Can form functional homotetrameric channels and heterotetrameric channels that contain variable proportions of KCNA1, KCNA2, KCNA4, KCNA5, KCNA6, KCNA7, and possibly other family members as well; channel properties depend on the type of alpha subunits that are part of the channel. Channel properties are modulated by cytoplasmic beta subunits that regulate the subcellular location of the alpha subunits and promote rapid inactivation of delayed rectifier potassium channels. In vivo, membranes probably contain a mixture of heteromeric potassium channel complexes, making it difficult to assign currents observed in intact tissues to any particular potassium channel family member. Homotetrameric KCNA1 forms a delayed-rectifier potassium channel that opens in response to membrane depolarization, followed by slow spontaneous channel closure. In contrast, a heterotetrameric channel formed by KCNA1 and KCNA4 shows rapid inactivation. Regulates neuronal excitability in hippocampus, especially in mossy fibers and medial perforant path axons, preventing neuronal hyperexcitability. May function as down-stream effector for G protein-coupled receptors and inhibit GABAergic inputs to basolateral amygdala neurons. May contribute to the regulation of neurotransmitter release, such as gamma-aminobutyric acid (GABA) release. Plays a role in regulating the generation of action potentials and preventing hyperexcitability in myelinated axons of the vagus nerve, and thereby contributes to the regulation of heart contraction. Required for normal neuromuscular responses. Regulates the frequency of neuronal action potential firing in response to mechanical stimuli, and plays a role in the perception of pain caused by mechanical stimuli, but does not play a role in the perception of pain due to heat stimuli. Required for normal responses to auditory stimuli and precise location of sound sources, but not for sound perception. The use of toxins that block specific channels suggest that it contributes to the regulation of the axonal release of the neurotransmitter dopamine. Required for normal postnatal brain development and normal proliferation of neuronal precursor cells in the brain. Plays a role in the reabsorption of Mg(2+) in the distal convoluted tubules in the kidney and in magnesium ion homeostasis, probably via its effect on the membrane potential.
-
基因功能参考文献:
- KCNQ activation decreased seizure latency by >/=50% in Kcnq1 strain mice but had no effect in the Kcna1 strain. However, in simultaneous EEG and ECG recordings, KCNQ activation significantly reduced spontaneous seizure frequency in Kcna1-/- mice by ~60%. In Kcnq1 mice, KCNQ activation produced adverse cardiac effects including profound bradycardia and abnormal increases in heart rate variability and AV conduction blocks. PMID: 29265344
- Kcna1(-/-) mice, a model of sudden unexpected death in epilepsy, experienced an increase in basal respiratory drive, chronic oxygen desaturation, frequent apnea-hypopnea (A-H), an atypical breathing sequence of A-H-tachypnea-A-H, increased tidal volume, and hyperventilation induced by methacholine. PMID: 29327348
- the pore-forming subunit of the large conductance voltage and calcium-activated potassium (BK, Slo1, or KCa1.1) channels encoded by a single KCa1.1 gene assembles in a fourfold symmetric fashion. Functional diversity arises from two families of regulatory subunits, beta and gamma, which help define the range of voltages over which BK channels in a given cell are activated, thereby defining physiological roles PMID: 30224470
- The results of this study suggest that the accumulated rest deficiency is associated with sudden death in Kv1.1 KO mice. PMID: 29193044
- No evidence has been found for developmental compensation of inherited Kv1.1 dysfunction in a mouse model of presynaptic channelopathy. PMID: 27381274
- Kv1.1 defects abolish presynaptic spike width modulation by subthreshold somatic depolarization. PMID: 28193892
- Low-voltage-activated K(+) (gKL) and hyperpolarization-activated mixed cation conductances (gh) mediate currents, IKL and Ih, through channels of the Kv1 (KCNA) and HCN families respectively and give auditory neurons the temporal precision required for signaling information about the onset, fine structure, and time of arrival of sounds. PMID: 28065805
- we identify Kvbeta1.1 as a sensor of pyridine nucleotide changes and as a modulator of Kv4.2 gating, action potential duration, and ECG in the mouse heart. PMID: 27986658
- This study provide new insights into the dynamic and differential distribution of Kv1 channels and associated proteins during myelination. PMID: 26840208
- age-associated changes in Sphingolipid composition or CerS2 ablation upregulate K(Ca) 1.1 and impair Ca(2+) mobilization, which thereby induces contractile dysfunction of gastric smooth muscle. PMID: 26288989
- Kcna-1 null mice initially expressed only a few of the most severe seizure types that progressively increased in frequency and decreased in seizure severity PMID: 26724401
- Kv1.2 channels represent an important physiological link in electric field-induced cell migration. PMID: 26580832
- Spontaneous seizures in Kcna1-null mice activate Fos expression in select limbic circuits PMID: 26112121
- Data suggest that the behavioral effect of Kv1.1 deletion is primarily to impede binaural integration and thus to mimic monaural hearing. PMID: 25602577
- The Kv1.1 null mouse is a potential model for sudden unexpected death in epilepsy in patients PMID: 25377007
- Kv1.1 is modulated by ANK3 in conditions of high dietary magnesium PMID: 23903368
- heterozygous mice subjected to P6 hypoxia exhibit increased susceptibility to flurothyl-induced seizures PMID: 24032507
- these data indicate that loss of Kv1.1 enhances synaptic release in the CA3 region, which reduces spike timing precision of individual neurons leading to disorganization of network oscillatory activity and promotes the emergence of fast ripples. PMID: 23466697
- Kv1.1 acts as a mechanosensitive brake that regulates mechanical sensitivity of fibers associated with mechanical perception PMID: 23473320
- It was concluded that Kv1.1-deficiency causes hyperexcitability in large myelinated axons in vagus nerve which could contribute to autonomic dysfunction in Kcna1-null mice, and that KCNQ openers reveals synergy between Kv1 and KCNQ channels. PMID: 22641786
- This study points out that juxtaparanodal K(+) channels composed of Kv1.1 subunits exert an important role in dampening the excitability of motor nerve axons during fatigue or ischemic insult. PMID: 22609489
- mechanisms for processing acoustic transients less effective in Kcna1 -/- mice PMID: 22302114
- Overlapping patterns with differential expression and precise localization of Kv1.1 and Kv1.2 channels targeted to specialized subcellular compartments contribute to distinctive patterns of neuronal excitability --REVIEW PMID: 22612818
- Suprathreshold auditory evoked potentials coupled with their normal thresholds suggests that a disruption in central neural processing in Kcna1 null mice and not peripheral hearing loss is responsible for their poor sound localization. PMID: 22396426
- The BK channels in parotid acinar cells have a much more hyperpolarized voltage activation range than BK channels in most other cell types, attributable to an accessory protein, LRRC26, which is expressed in parotid glands. PMID: 21984254
- K(Ca)1.1-mediated K(+) secretion mainly occurs in the crypts of the murine distal colon. PMID: 21822598
- These results imply a fundamental role for Kv1.1 in temporal integration of excitation and inhibition during sound source localization. PMID: 21224222
- Kv1.1 channels are expressed in the beta-cells of several species PMID: 21483673
- Found that the shifted activation of parotid BK channels resulted from a hyperpolarizing shift of the voltage dependence of voltage sensor activation and channel opening and included a large change in the coupling of these two processes. PMID: 20519930
- data suggest that Kv1.1 deficiency leads to impaired neural control of cardiac rhythmicity due in part to aberrant parasympathetic neurotransmission, making Kcna1 a strong candidate gene for human sudden unexplained death in epilepsy PMID: 20392939
- In Kcna1-null mice the absence of the Kv1.1 subunit results in a loss of temporal fidelity (increased jitter) and the failure to follow high-frequency amplitude-modulated sound stimulation in vivo PMID: 14534254
- Lack of Kv1.1 potassium channel subunits in CA3 pyramidal cells leads to synaptic hyperexcitability, as reflected in the propensity of these cells to generate multiple action potentials. PMID: 14636320
- Mceph/mceph mice carry a deletion in the gene encoding the Shaker-like voltage-gated potassium channel subtype 1, Kcna1. This causes a frame shift and the predicted MCEPH protein is truncated at amino acid 230, terminating with six aberrant amino acids. PMID: 14686897
- MCEPH protein is expressed in the brain of mceph/mceph mice. MCEPH was found to lack mature (Golgi) glycosylation, but to be core glycosylated and trapped in the endoplasmic reticulum (ER). Interactions between MCEPH and other Kv1 subunits PMID: 16305740
- Dendrotoxin-K(DTX-K) caused the largest increases, latency and jitter in Kcna1(-/-) cells and in 3 nM DTX-K-treated cells were similar to each other but increased compared with positive cells. PMID: 16672305
- Total absence of Kv1.1 can induce excessive overgrowth of hippocampus and ventral cortex in mice with a BALB/cByJ background, while mice with one wild type Kv1.1 allele develop normal-sized brains. PMID: 17250763
- increasing membrane excitability by removing the Kcna1 gene, masked the absence epilepsy caused by a P/Q-type Ca(2+) channelopathy. PMID: 17982453
- The neural pathways encoding behaviorally relevant, rapid auditory temporal fluctuations are not limited by the absence of Kv1.1 expression. PMID: 18926893
- K(v)1.1 and K(v)1.2 were predominantly expressed in distinct EGC phenotypes. PMID: 19549557
显示更多
收起更多
-
相关疾病:A spontaneous mutation leading to a frameshift and truncation of Kcna2 causes megencephaly with a 25% increase of brain weight relative to wild-type. Especially the hippocampus shows increased proliferation of neurons and astrocytes, leading to increased brain volume (PubMed:17315199). Mutant mice appear normal at birth. After 3-4 weeks, they display low body weight, a subtle shakiness in their gait, a preference for a strange sitting position that is maintained for periods ranging from 30 seconds to several minutes, excessive lacrimation and acoustic startle hypersensitivity (PubMed:8995755, PubMed:21966978). The increase in the acoustic startle response is down-regulated by treatment with the anti-epileptic drug valproate (PubMed:21966978). Mutant mice display an abnormal electro-encephalogram with single spikes and waves, when anesthesized (PubMed:21966978). The electric activity of mossy cells from the dentate hilus region is altered and shows increased firing of action potentials, probably due to the absence of functional Kcna1 channels (PubMed:14686897). Heterozygotes show mechanical allodynia, but no increased sensitivity to heat (PubMed:23473320). Homozygotes show no alteration of the islet of Langerhans structure, of the basal levels of insulin secretion and blood glucose levels (PubMed:21483673). Compared to wild-type, they display moderately increased insulin secretion in response to a glucose stimulus (PubMed:21483673). Besides, the frequency of beta cell action potentials is increased (PubMed:21483673).
-
亚细胞定位:Cell membrane; Multi-pass membrane protein. Cell projection, axon. Membrane. Perikaryon. Cell projection, dendrite. Cell junction. Cell junction, synapse. Cytoplasmic vesicle. Endoplasmic reticulum. Cell junction, synapse, presynaptic cell membrane. Cell junction, synapse, presynapse.
-
蛋白家族:Potassium channel family, A (Shaker) (TC 1.A.1.2) subfamily, Kv1.1/KCNA1 sub-subfamily
-
组织特异性:Detected in brain. Detected in the juxtaparanodal regions of the nodes of Ranvier in myelinated axons. Detected in the paranodal region in sciatic nerve. Detected on cell bodies in cerebellum, dorsal and ventral cochlear nucleus, pontine reticular nucleus
-
数据库链接:
KEGG: mmu:16485
STRING: 10090.ENSMUSP00000055225
UniGene: Mm.40424
Most popular with customers
-
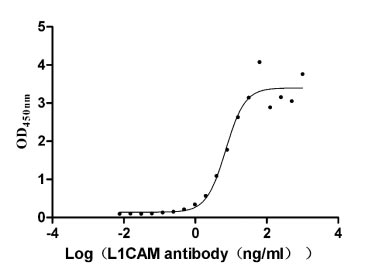
Recombinant Human Neural cell adhesion molecule L1 (L1CAM), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Mouse GDNF family receptor alpha-like (Gfral), partial (Active)
Express system: Mammalian cell
Species: Mus musculus (Mouse)
-
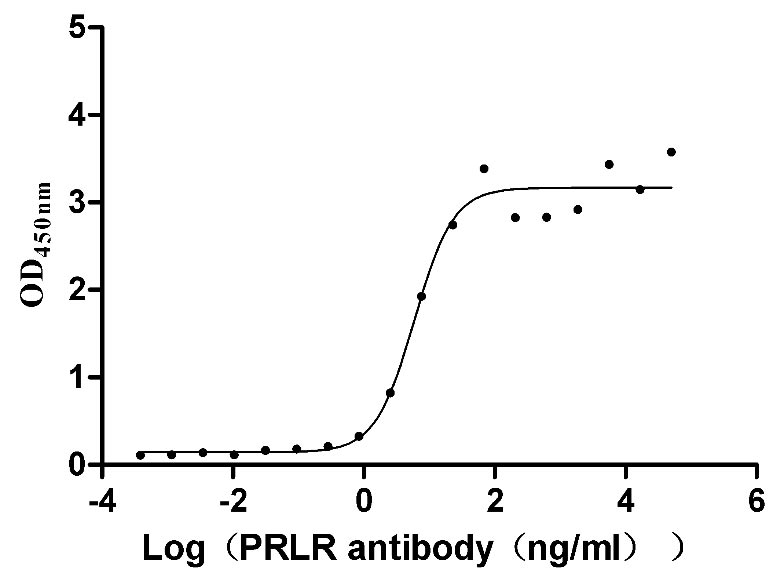
Recombinant Mouse Prolactin receptor (Prlr), partial (Active)
Express system: Mammalian cell
Species: Mus musculus (Mouse)
-
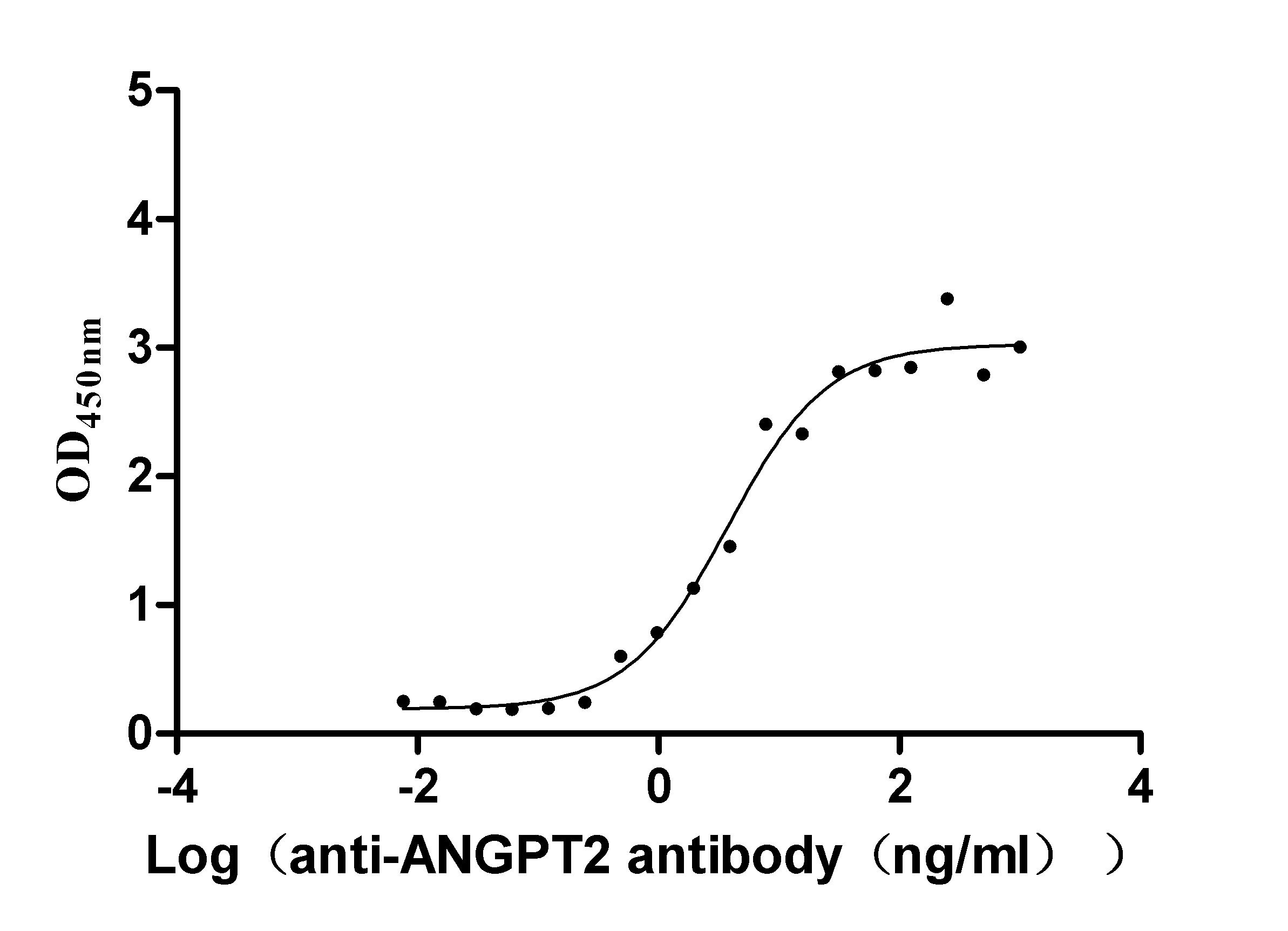
Recombinant Human Angiopoietin-2 (ANGPT2) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Dog Angiopoietin-2 (ANGPT2) (Active)
Express system: Mammalian cell
Species: Canis lupus familiaris (Dog) (Canis familiaris)
-
Recombinant Mouse Claudin-18 (Cldn18)-VLPs (Active)
Express system: Mammalian cell
Species: Mus musculus (Mouse)
-
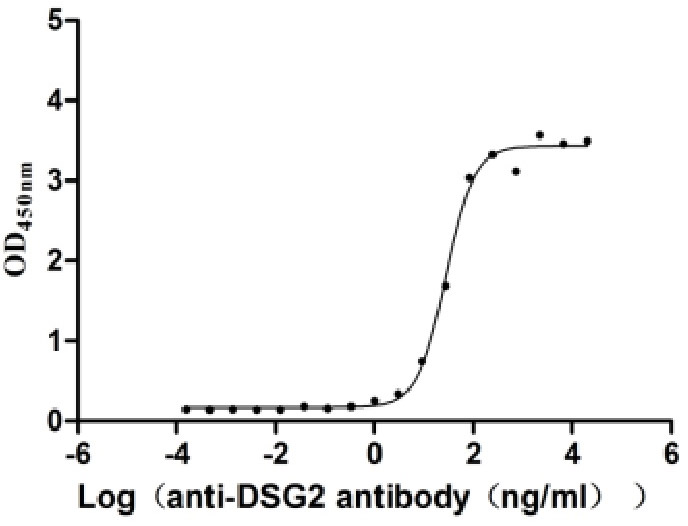
Recombinant Human Desmoglein-2 (DSG2), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
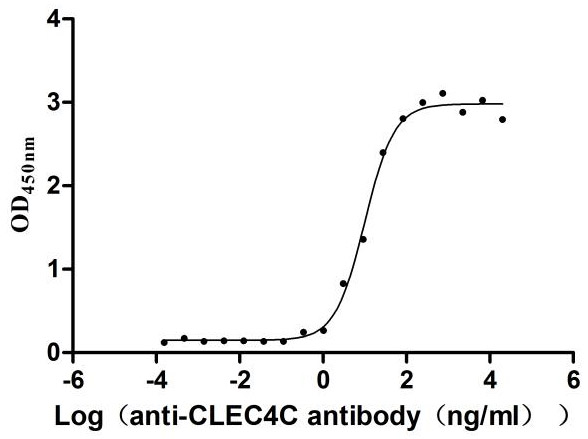
Recombinant Human C-type lectin domain family 4 member C (CLEC4C), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)


-AC1.jpg)
-AC1.jpg)