Recombinant Sindbis virus Structural polyprotein, partial
-
中文名称:Recombinant Sindbis virus Structural polyprotein,partial,Yeast
-
货号:CSB-YP361018SHZ1
-
规格:
-
来源:Yeast
-
其他:
-
中文名称:Recombinant Sindbis virus Structural polyprotein,partial,Yeast
-
货号:CSB-EP361018SHZ1
-
规格:
-
来源:E.coli
-
其他:
-
中文名称:Recombinant Sindbis virus Structural polyprotein,partial,Yeast
-
货号:CSB-EP361018SHZ1-B
-
规格:
-
来源:E.coli
-
共轭:Avi-tag Biotinylated
E. coli biotin ligase (BirA) is highly specific in covalently attaching biotin to the 15 amino acid AviTag peptide. This recombinant protein was biotinylated in vivo by AviTag-BirA technology, which method is BriA catalyzes amide linkage between the biotin and the specific lysine of the AviTag.
-
其他:
-
中文名称:Recombinant Sindbis virus Structural polyprotein,partial,Yeast
-
货号:CSB-BP361018SHZ1
-
规格:
-
来源:Baculovirus
-
其他:
-
中文名称:Recombinant Sindbis virus Structural polyprotein,partial,Yeast
-
货号:CSB-MP361018SHZ1
-
规格:
-
来源:Mammalian cell
-
其他:
产品详情
-
纯度:>85% (SDS-PAGE)
-
基因名:N/A
-
Uniprot No.:
-
别名:Structural polyprotein; p130
-
种属:Sindbis virus (SINV)
-
蛋白长度:Partial
-
蛋白标签:Tag type will be determined during the manufacturing process.
The tag type will be determined during production process. If you have specified tag type, please tell us and we will develop the specified tag preferentially. -
产品提供形式:Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
复溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
储存条件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保质期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
货期:Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事项:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet :Please contact us to get it.
相关产品
靶点详情
-
功能:Forms an icosahedral capsid with a T=4 symmetry composed of 240 copies of the capsid protein surrounded by a lipid membrane through which penetrate 80 spikes composed of trimers of E1-E2 heterodimers. The capsid protein binds to the viral RNA genome at a site adjacent to a ribosome binding site for viral genome translation following genome release. Possesses a protease activity that results in its autocatalytic cleavage from the nascent structural protein. Following its self-cleavage, the capsid protein transiently associates with ribosomes, and within several minutes the protein binds to viral RNA and rapidly assembles into icosahedric core particles. The resulting nucleocapsid eventually associates with the cytoplasmic domain of the spike glycoprotein E2 at the cell membrane, leading to budding and formation of mature virions. In case of infection, new virions attach to target cells and after clathrin-mediated endocytosis their membrane fuses with the host endosomal membrane. This leads to the release of the nucleocapsid into the cytoplasm, followed by an uncoating event necessary for the genomic RNA to become accessible. The uncoating might be triggered by the interaction of capsid proteins with ribosomes. Binding of ribosomes would release the genomic RNA since the same region is genomic RNA-binding and ribosome-binding. Specifically inhibits interleukin-1 receptor-associated kinase 1/IRAK1-dependent signaling during viral entry, representing a means by which the alphaviruses may evade innate immune detection and activation prior to viral gene expression.; Provides the signal sequence for the translocation of the precursor of protein E3/E2 to the host endoplasmic reticulum. Furin-cleaved E3 remains associated with spike glycoprotein E1 and mediates pH protection of the latter during the transport via the secretory pathway. After virion release from the host cell, the assembly protein E3 is gradually released in the extracellular space.; Plays an essential role in viral attachment to target host cell, by binding to the cell receptor. Synthesized as a pE2 precursor which is processed by furin at the cell membrane just before virion budding, giving rise to E2-E1 heterodimer. The pE2-E1 heterodimer is stable, whereas E2-E1 is unstable and dissociate at low pH. pE2 is processed at the last step, presumably to avoid E1 fusion activation before its final export to cell surface. E2 C-terminus contains a transitory transmembrane that would be disrupted by palmitoylation, resulting in reorientation of the C-terminal tail from lumenal to cytoplasmic side. This step is critical since E2 C-terminus is involved in budding by interacting with capsid proteins. This release of E2 C-terminus in cytoplasm occurs lately in protein export, and precludes premature assembly of particles at the endoplasmic reticulum membrane.; Protein 6K: Acts as a viroporin that participates in virus glycoprotein processing, cell permeabilization and budding of viral particles. Disrupts the calcium homeostasis of the cell, probably at the endoplasmic reticulum level resulting in the increased levels of cytoplasmic calcium. Because of its lipophilic properties, the 6K protein is postulated to influence the selection of lipids that interact with the transmembrane domains of the glycoproteins, which, in turn, affects the deformability of the bilayer required for the extreme curvature that occurs as budding proceeds. Present in low amount in virions, about 3% compared to viral glycoproteins.; Class II viral fusion protein. Fusion activity is inactive as long as E1 is bound to E2 in mature virion. After virus attachment to target cell and endocytosis, acidification of the endosome would induce dissociation of E1/E2 heterodimer and concomitant trimerization of the E1 subunits. This E1 trimer is fusion active, and promotes release of viral nucleocapsid in cytoplasm after endosome and viral membrane fusion. Efficient fusion requires the presence of cholesterol and sphingolipid in the target membrane.
-
基因功能参考文献:
- The s conclude that H230 of E1 protein is essential for the assembly of complete infectious Sindbis virus virions and that the presence of an amino acid at E2 position 209 is required for complete budding of Sindbis virus particles although several different amino acids can be at this location without affecting the titer. PMID: 27412592
- Mutating conserved cysteines in the e2 glycoprotein causes virus-specific assembly defects. PMID: 22238319
- Interactions of the cytoplasmic domain of E2 with nucleocapsid cores promote alphavirus budding. PMID: 22190727
- analysis of molecular links between the E2 envelope glycoprotein and nucleocapsid core in Sindbis virus PMID: 22001018
- Interaction of E2 glycoprotein with heparan sulfate is crucial for cellular infection of Sindbis virus PMID: 20300181
- Xray structure of spike, representing an intermediate in fusion process and clarifying maturation process; trimer of E2-E1 is similar to spikes in the neutral pH virus; amino- and carboxy-terminal domains of E2 form immunoglobulin-like folds PMID: 21124457
- specific amino acids within the region comprising amino acids 81 to 113 responsible for the encapsidation of the genomic RNA have been identified. PMID: 18305029
- The data indicate that the role of the cysteine residues in E3 is not primarily structural: it is hypothesized that E3 has an enzymatic or functional role in virus assembly, and these possibilities are further discussed. PMID: 19109378
显示更多
收起更多
-
亚细胞定位:[Capsid protein]: Virion. Host cytoplasm. Host cell membrane. Host nucleus.; [Precursor of protein E3/E2]: Virion membrane; Single-pass type I membrane protein. Host cell membrane; Single-pass type I membrane protein.; [Spike glycoprotein E2]: Virion membrane; Single-pass type I membrane protein. Host cell membrane; Single-pass type I membrane protein.; [6K protein]: Host cell membrane; Multi-pass membrane protein. Virion membrane; Multi-pass membrane protein.; [Spike glycoprotein E1]: Virion membrane; Single-pass type I membrane protein. Host cell membrane; Single-pass type I membrane protein.
-
蛋白家族:Alphavirus structural polyprotein family
-
数据库链接:
KEGG: vg:1502155
Most popular with customers
-
Recombinant Human Claudin-18.2 (CLDN18.2)-VLPs (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
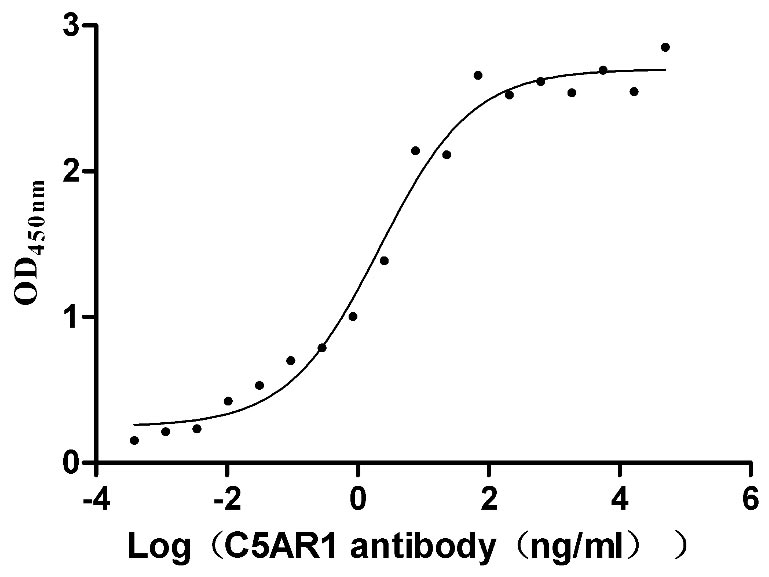
Recombinant Human C5a anaphylatoxin chemotactic receptor 1 (C5AR1)-VLPs (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
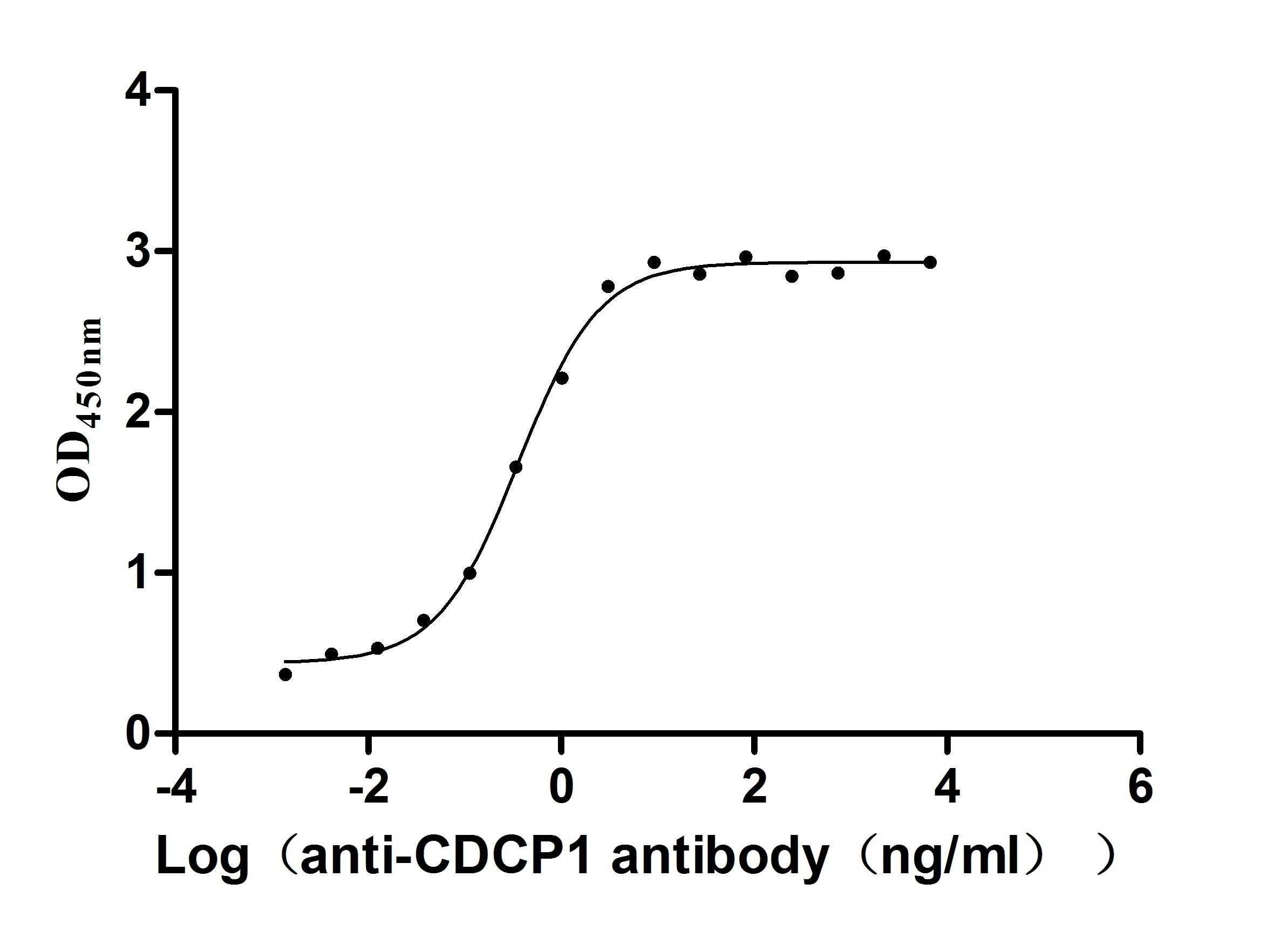
Recombinant Human CUB domain-containing protein 1 (CDCP1), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
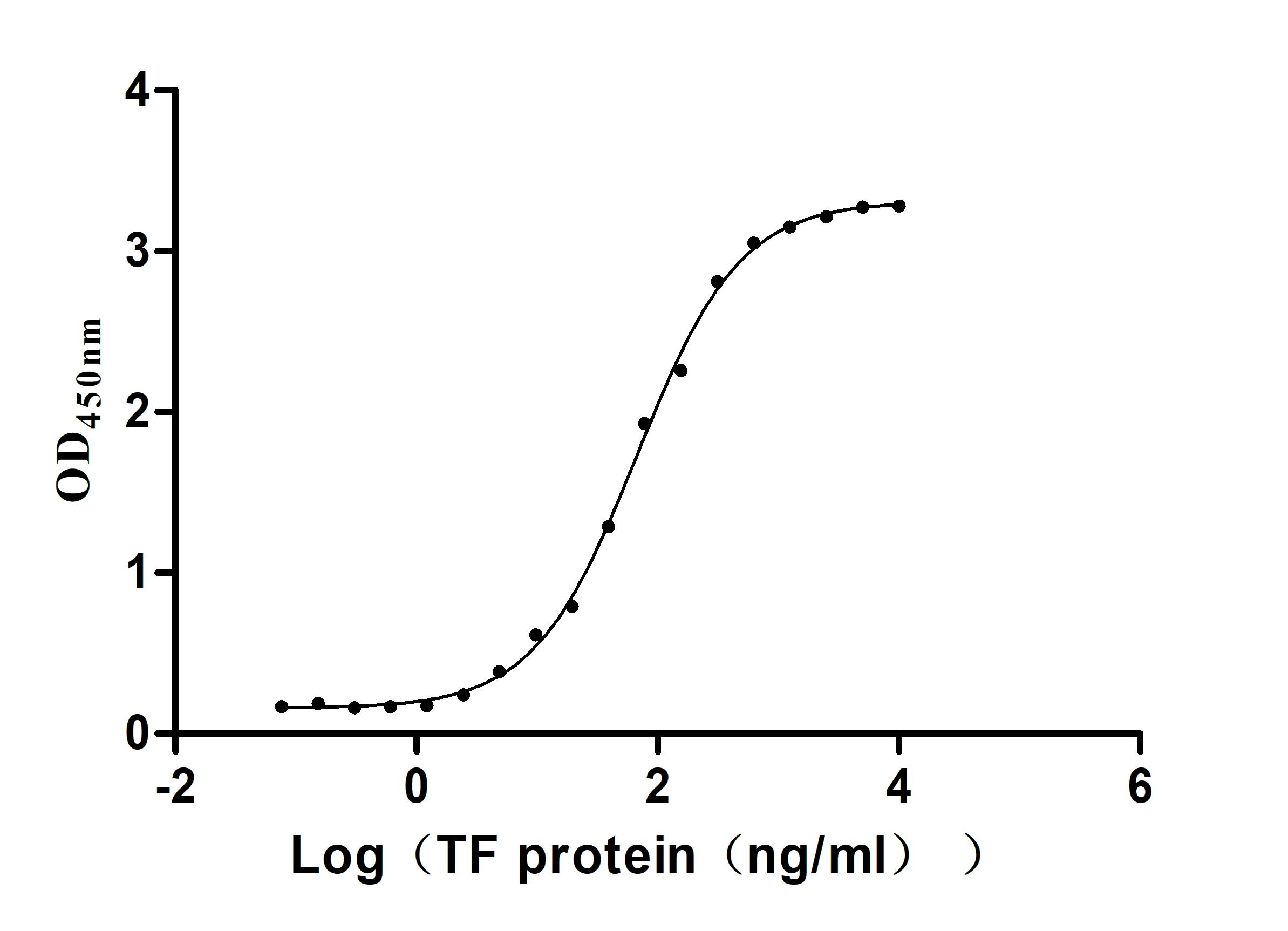
Recombinant Human Serotransferrin(TF) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
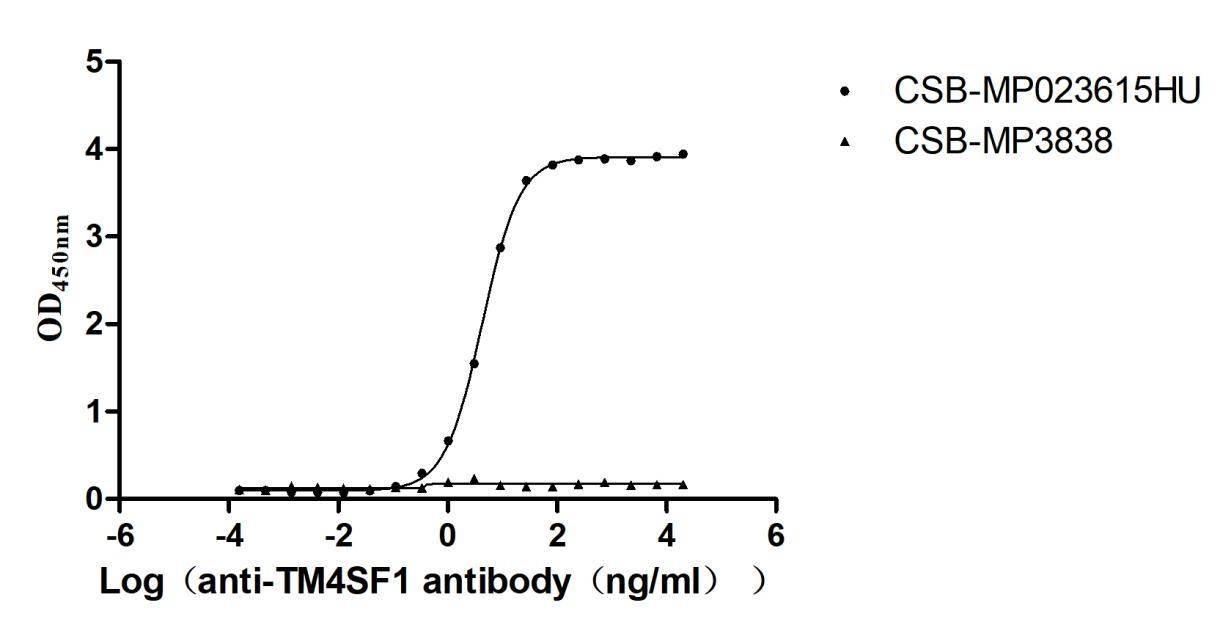
Recombinant Human Transmembrane 4 L6 family member 1(TM4SF1)-VLPs (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
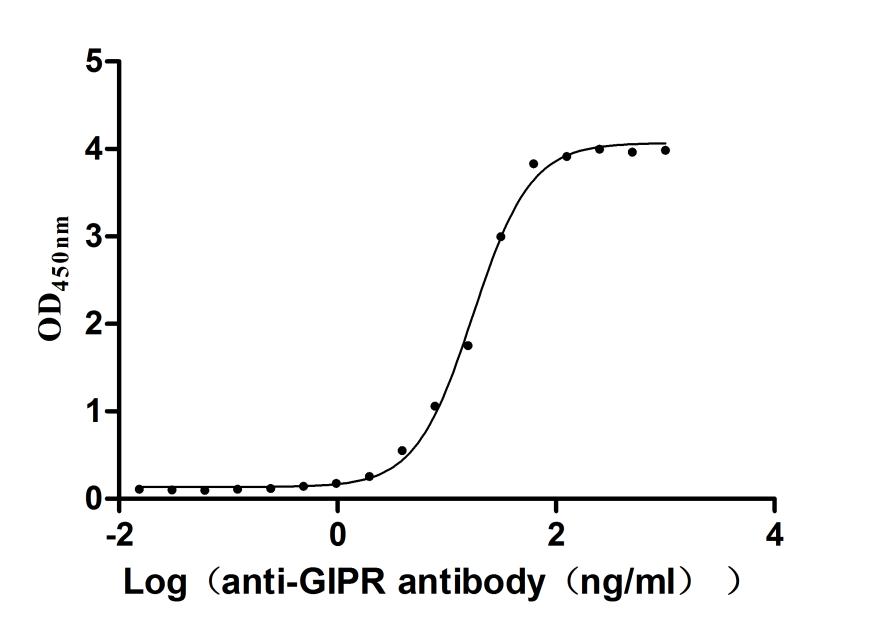
Recombinant Human Gastric inhibitory polypeptide receptor(GIPR),partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
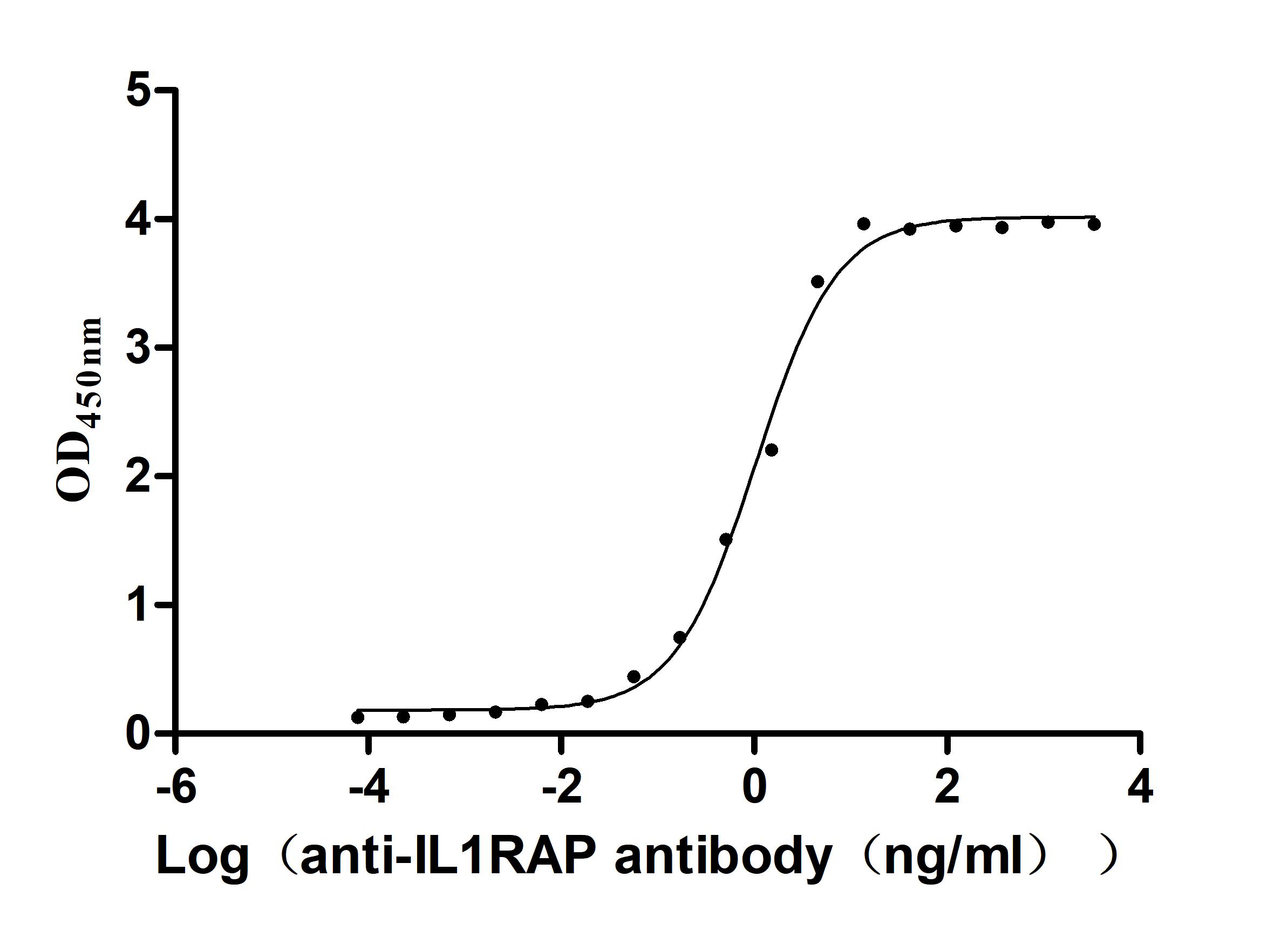
Recombinant Macaca fascicularis Interleukin 1 receptor accessory protein(IL1RAP), partial (Active)
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)


-AC1.jpg)