Recombinant Saccharomyces cerevisiae Heat shock protein SSA1 (SSA1), partial
In Stock-
中文名称:酿酒酵母SSA1重组蛋白
-
货号:CSB-EP319915SVG
-
规格:¥1836
-
图片:
-
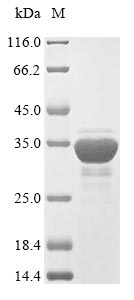
(Tris-Glycine gel) Discontinuous SDS-PAGE (reduced) with 5% enrichment gel and 15% separation gel.
-
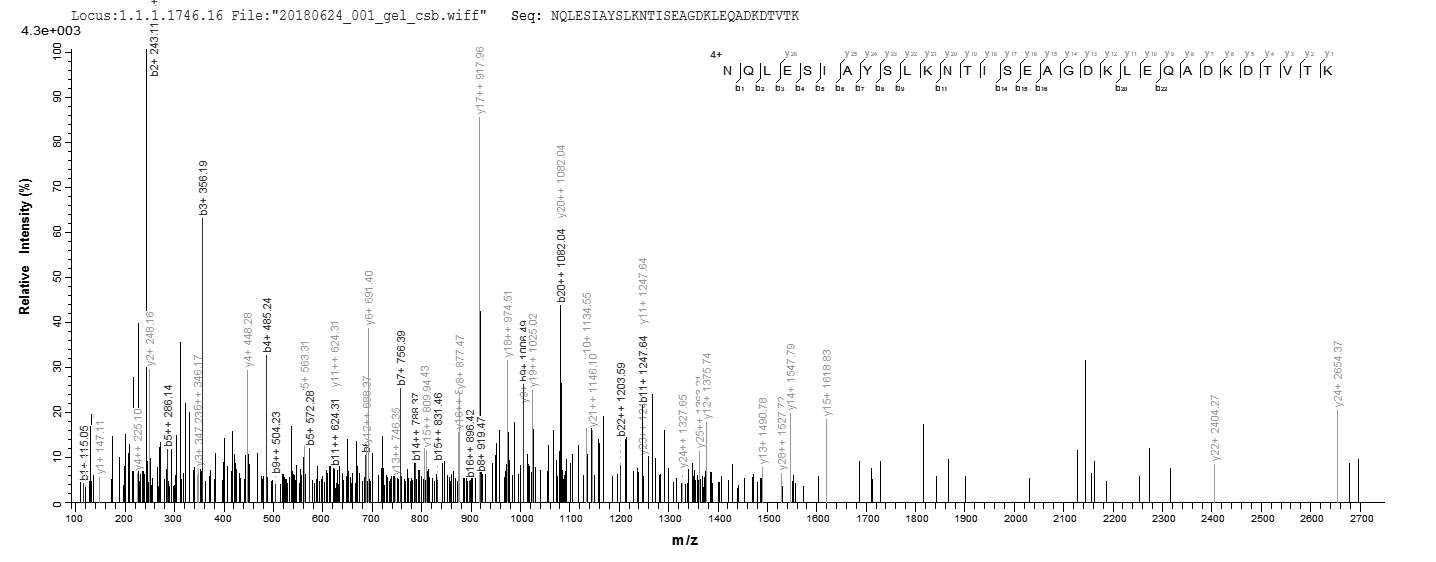
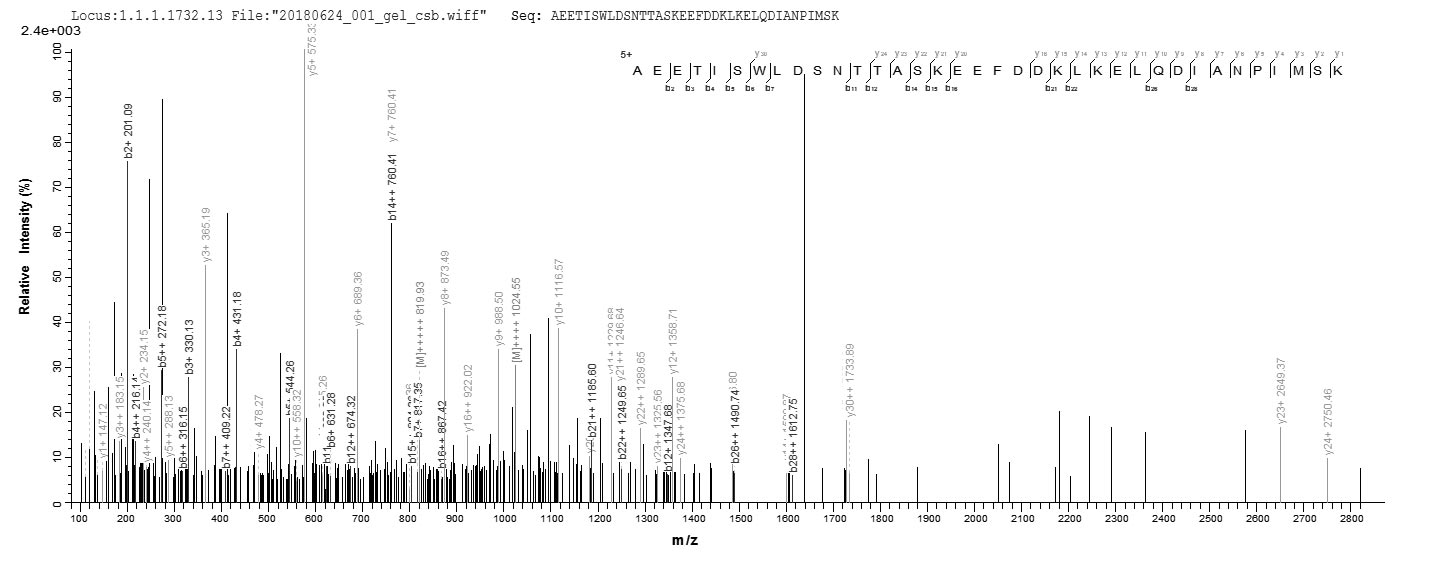
Based on the SEQUEST from database of E.coli host and target protein, the LC-MS/MS Analysis result of CSB-EP319915SVG could indicate that this peptide derived from E.coli-expressed Saccharomyces cerevisiae (strain ATCC 204508 / S288c) (Baker's yeast) SSA1.
-
Based on the SEQUEST from database of E.coli host and target protein, the LC-MS/MS Analysis result of CSB-EP319915SVG could indicate that this peptide derived from E.coli-expressed Saccharomyces cerevisiae (strain ATCC 204508 / S288c) (Baker's yeast) SSA1.
-
-
其他:
产品详情
-
纯度:Greater than 85% as determined by SDS-PAGE.
-
基因名:SSA1
-
Uniprot No.:
-
别名:SSA1; YAL005C; Heat shock protein SSA1; Heat shock protein YG100
-
种属:Saccharomyces cerevisiae (strain ATCC 204508 / S288c) (Baker's yeast)
-
蛋白长度:Partial
-
来源:E.coli
-
分子量:28.3 kDa
-
表达区域:443-642aa
-
氨基酸序列ERAKTKDNNLLGKFELSGIPPAPRGVPQIEVTFDVDSNGILNVSAVEKGTGKSNKITITNDKGRLSKEDIEKMVAEAEKFKEEDEKESQRIASKNQLESIAYSLKNTISEAGDKLEQADKDTVTKKAEETISWLDSNTTASKEEFDDKLKELQDIANPIMSKLYQAGGAPGGAAGGAPGGFPGGAPPAPEAEGPTVEEVD
Note: The complete sequence including tag sequence, target protein sequence and linker sequence could be provided upon request. -
蛋白标签:N-terminal 10xHis-tagged and C-terminal Myc-tagged
-
产品提供形式:Liquid or Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
缓冲液:Tris-based buffer,50% glycerol
-
储存条件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保质期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
货期:3-7 business days
-
注意事项:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet & COA:Please contact us to get it.
相关产品
靶点详情
-
功能:May play a role in the transport of polypeptides both across the mitochondrial membranes and into the endoplasmic reticulum. A functional difference between SSA1 and SSA2 proteins is expected. SSA1 can participate in the ATP-dependent disassembly of clathrin-coated vesicles.
-
基因功能参考文献:
- Hsp90 (Hsp82) and yeast Hsp70 (Ssa1), directly interact in vitro in the absence of the yeast Hop homolog (Sti1), and identify a region in the middle domain of yeast Hsp90 that is required for the interaction. PMID: 29463764
- Studied deletions of two major chaperone proteins, SSA1 and SSB1, from the HSP70 chaperone network in Sacchromyces cerevisiae. PMID: 25689132
- yeast expressing P417L or P417S as the only copy of Ssa were temperature sensitive and exhibited defects in Ssa1-dependent protein translocation and misfolded protein degradation. PMID: 25913688
- Nearly all interactions remained unchanged or decreased after DNA damage, but 5 proteins increased interactions with Ssa1 and/or Hsp82, including the ribonucleotide reductase (RNR) subunit Rnr4. PMID: 25452130
- The major ubiquitin ligase targeting the superfluous Fas2 subunit to the proteasome is Ubr1. The ubiquitin-conjugating enzymes Ubc2 and Ubc4 assist the degradation process. PMID: 25564609
- Ssa1p and Swa2p cooperatively disassemble yeast clathrin coat baskets PMID: 23913685
- The Hsp70 Chaperone Ssa1 and the AAA-Type ATPase Cdc48 Are Required for Ubr1-Dependent ERAD of Ste6*. PMID: 23988329
- Data indicate that the Hsp70, Ssa1p, facilitates an interaction between a novel misfolded substrate and San1p. PMID: 23653356
- Sis1 and Hsp70 operate sequentially with the quality control E3 ubiquitin ligase Ubr1 to target short-lived green fluorescent protein for degradation. PMID: 23341891
- findings demonstrate that Hsp70 is a proximal sensor for Hsf1-mediated cytoprotection and can discriminate between two distinct environmental stressors PMID: 22809627
- T36 phosphorylation triggers displacement of Ydj1, allowing Ssa1 to bind the G1 cyclin Cln3 and promote its degradation; these results establish an active role for Hsp70 chaperones as signal transducers mediating growth control of G1 cyclin abundance and activity. PMID: 23217712
- the client binding domain of Hsc70 and Ssa1p binds two regions within alpha-Syn similar to a tweezer, with the first spanning residues 10-45 and the second spanning residues 97-102. PMID: 22843682
- a role for Ssa1 in mediating localization of nascent peptide-ribosome-mRNA complexes to the mitochondria PMID: 22138184
- Conformation-dependent Ssa1 hydrophobicity and aggregation play a key role in Ssa1 function. PMID: 20835845
- observations strongly suggest that lysine 339 and its flanking amino acid stretches are involved in the interaction between Ure2p and Ssa1p PMID: 21078122
- Ssa1 plays a general role in elimination of gluconeogenic enzymes. PMID: 20513352
- a potent cochaperone of Ssa1 is Cns1 PMID: 15044454
- Stimulates prion formation and polymer growth by stabilizing misfolded proteins. PMID: 15545639
- Sis1 has a bipartite interaction with Ssa in vivo PMID: 15687271
- Data suggest that Ssa1-21p interferes with disruption of large Sup35p aggregates, which lack or have limited capacity to function as seed, into polymers that function more efficiently as [PSI+] seeds. PMID: 15701791
- characterized the influence of Hsp104 and Ssa1 on the disassembly of Hsp26 x substrate complexes in vitro and in vivo PMID: 15843375
- SSB/SSE and SSA/SSE transiently associate with newly synthesized polypeptides with different kinetics PMID: 16219770
- Sse1 functionally interacts with the Hsp70 chaperones Ssa and Ssb PMID: 16221677
- A structural basis for the regulation of heat-shock proteins in S. cerevisiae is presented. PMID: 16737444
- The introduction of Ssa1p improves secretory proteins in Pichia pastoris secretion 4-7 times. PMID: 16889384
- Jjj1, when overexpressed, is able to partially substitute for the Zuo1:Ssb chaperone machinery by recruiting Ssa to the ribosome, facilitating its interaction with nascent polypeptide chains PMID: 17242366
- Stability experiments revealed that only Hsp70 proteins Ydj1-protected can hydrolyze ATP under prolonged stress. PMID: 17985367
- To determine how the mutations alter Hsp70 we analyzed biochemically the substrate-binding domain (SBD) mutant L483W and the nucleotide-binding domain (NBD) mutants A17V and R34K. PMID: 18706386
- Sse1 exploits a Bag-1-like mechanism to catalyze nucleotide release, which involves opening of the Ssa1 NBD by tilting lobe II. Hsp110 proteins use a unique binding mode to catalyze nucleotide release from Hsp70s by a functionally convergent mechanism PMID: 18948593
- These results suggest that cytosolic Hsp70 plays multiple roles in TBSV replication, such as affecting the subcellular localization and membrane insertion of the viral replication proteins as well as the assembly of the viral replicase. PMID: 19153242
- An in vitro replicase assembly assay with Ssa1p(ts) revealed that functional Ssa1p is required during the replicase assembly process, but not during minus- or plus-strand synthesis of Tombusvirus. PMID: 19748649
显示更多
收起更多
-
亚细胞定位:Cytoplasm. Secreted, cell wall.
-
蛋白家族:Heat shock protein 70 family
-
数据库链接:
KEGG: sce:YAL005C
STRING: 4932.YAL005C
Most popular with customers
-
Recombinant Human Leukemia inhibitory factor (LIF) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Delta-like protein 3 (DLL3), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Interleukin-17A (IL17A) (T26A) (Active)
Express system: Baculovirus
Species: Homo sapiens (Human)
-
Recombinant Mouse Cell adhesion molecule 1 (Cadm1), partial (Active)
Express system: Mammalian cell
Species: Mus musculus (Mouse)
-
Recombinant Human Interleukin-2 receptor subunit alpha (IL2RA), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Rat Gastric inhibitory polypeptide receptor (Gipr), partial (Active)
Express system: Mammalian cell
Species: Rattus norvegicus (Rat)



-AC1.jpg)