Recombinant Rat Calmodulin-1 (Calm1)
In Stock-
中文名称:Recombinant Rat Calmodulin-1(Calm1)
-
货号:CSB-EP004445RA
-
规格:¥1344
-
图片:
-
(Tris-Glycine gel) Discontinuous SDS-PAGE (reduced) with 5% enrichment gel and 15% separation gel.
-
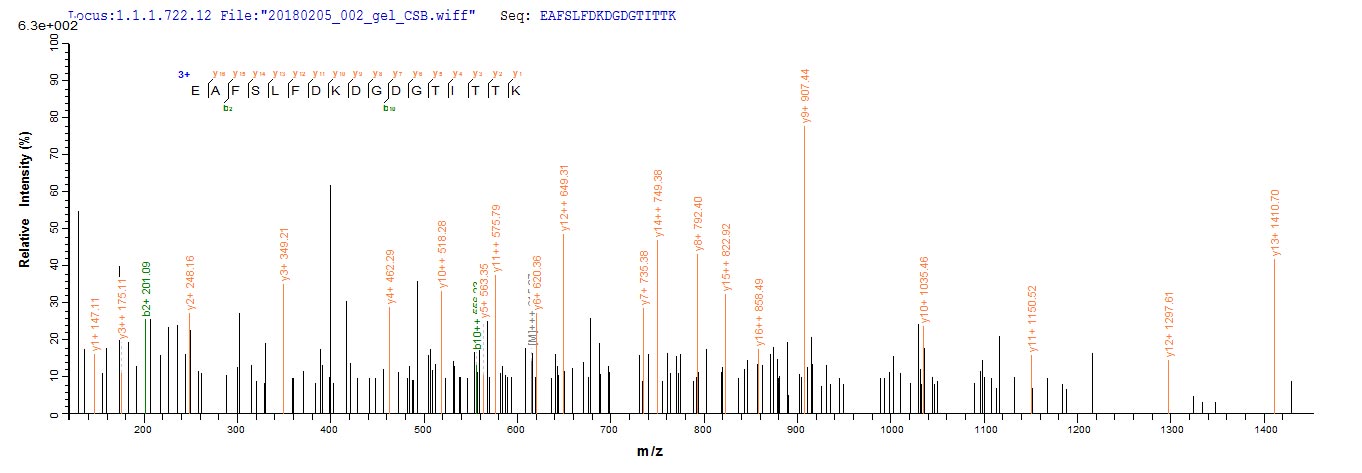
Based on the SEQUEST from database of E.coli host and target protein, the LC-MS/MS Analysis result of CSB-EP004445RA could indicate that this peptide derived from E.coli-expressed Rattus norvegicus (Rat) Calm1.
-
Based on the SEQUEST from database of E.coli host and target protein, the LC-MS/MS Analysis result of CSB-EP004445RA could indicate that this peptide derived from E.coli-expressed Rattus norvegicus (Rat) Calm1.
-
-
其他:
产品详情
-
纯度:Greater than 85% as determined by SDS-PAGE.
-
基因名:
-
Uniprot No.:
-
别名:Calm1; Calm; Cam; Cam1; CaMICalmodulin-1
-
种属:Rattus norvegicus (Rat)
-
蛋白长度:Full Length of Mature Protein
-
来源:E.coli
-
分子量:34.2 kDa
-
表达区域:2-149aa
-
氨基酸序列ADQLTEEQIAEFKEAFSLFDKDGDGTITTKELGTVMRSLGQNPTEAELQDMINEVDADGNGTIDFPEFLTMMARKMKDTDSEEEIREAFRVFDKDGNGYISAAELRHVMTNLGEKLTDEEVDEMIREADIDGDGQVNYEEFVQMMTAK
Note: The complete sequence including tag sequence, target protein sequence and linker sequence could be provided upon request. -
蛋白标签:N-terminal 6xHis-SUMO-tagged and C-terminal Myc-tagged
-
产品提供形式:Liquid or Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
缓冲液:Tris-based buffer,50% glycerol
-
储存条件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保质期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
货期:3-7 business days
-
注意事项:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet & COA:Please contact us to get it.
相关产品
靶点详情
-
功能:Calmodulin mediates the control of a large number of enzymes, ion channels, aquaporins and other proteins through calcium-binding. Among the enzymes to be stimulated by the calmodulin-calcium complex are a number of protein kinases and phosphatases. Together with CCP110 and centrin, is involved in a genetic pathway that regulates the centrosome cycle and progression through cytokinesis. Is a regulator of voltage-dependent L-type calcium channels. Mediates calcium-dependent inactivation of CACNA1C. Positively regulates calcium-activated potassium channel activity of KCNN2. Forms a potassium channel complex with KCNQ1 and regulates electrophysiological activity of the channel via calcium-binding. Acts as a sensor to modulate the endomplasmic reticulum contacts with other organelles mediated by VMP1:ATP2A2.
-
基因功能参考文献:
- Tau could inhibit the H2O2-induced decrease in CamKII and CaM expression at both the mRNA and protein levels. PMID: 29279520
- These results suggest a connection between Ca(2+)-signaling via excitation-contraction coupling and the regulation of STARS-mediated gene expression in muscles. PMID: 27132186
- Hydrogen peroxide reduces calmodulin binding to RYR2 in rat cardiomyocytes. PMID: 26092277
- Disruption of calmodulin binding to KCNQ2 also impairs enrichment of heteromeric KCNQ2/KCNQ3 channels at the axonal surface by blocking their trafficking from the endoplasmic reticulum to the axon. PMID: 25077630
- Study reveals that apoCaM itself prominently regulates both voltage-gated Ca2+ and Na channels; ApoCaM binding to these channels enhances opening several-fold, matching the strongest forms of ion-channel regulation. PMID: 25417111
- The molecular events underlying the association between CaM and Kv7.2 and their regulation by Ca(2+), was examined. PMID: 24489773
- The study proposes that the structural basis of calcineurin activation by calmodulin is through displacement of the disordered fragment of the autoinhibitory domain which otherwise impedes active site access. PMID: 24018048
- Recombinant small (SK2) calcium channel and calmodulin bind with three different stoichiometries that depend on the molar ratio of 2SKp/2CaM in solution. PMID: 24420768
- CK2-mediated phosphorylation of calmodulin regulates the M-current, which is tonically regulated by CK2 and PP1 anchored to the KCNQ2 channel complex. PMID: 24627475
- GRK5 nuclear translocation downstream of select Gq-activating hypertrophic ligands is a calmodulin-dependent process PMID: 23472081
- Structural basis for the association of MAP6 protein with microtubules and its regulation by calmodulin. PMID: 23831686
- Data indicate that the two distinct CaM/OLFp complexes existed simultaneously with stable structures. PMID: 22877078
- structures of intact calmodulin (CaM)-free and CaM-bound endothelial nitric oxide synthase (eNOS) PMID: 23266515
- Sustained Epac activation induces a strong positive inotropic effect relating to enhanced calcium signaling and increased expression of calmodulin. PMID: 22910094
- Neurogranin targets calmodulin and lowers the threshold for the induction of long-term potentiation. PMID: 22848456
- The crystal structure of a CaM.Orai1-calmodulin binding domain complex, is reported. PMID: 23109337
- Cx32 is differentially phosphorylated and exists in a complex with SAP97 and CaM. PMID: 22718765
- Calmodulin bound to the first IQ motif is responsible for calcium-dependent regulation of myosin 5a. PMID: 22437832
- In PMCA-suppressed lines total CaM increased, and the calm I and calm II genes appeared to be responsible for this effect PMID: 21912933
- Calmodulin facilitates endocytosis in an activity-dependent manner. PMID: 22184217
- PKC and CaM protein expressions were downregulated in the hippocampus of neonatal rats exposed to lead. PMID: 19358756
- Both the location and orientation of CaM binding on the RyR2 are very similar to the skeletal muscle RyR1 isoform. PMID: 22067155
- PKC and CaM mRNA expression was downregulated in the hippocampus of baby rats with chronic lead exposure. PMID: 18761789
- molecular mechanisms of the phosphorylation-dependent regulation of NHE1 PMID: 21931166
- The present findings provide new insights on how MA interacts with CaM that may ultimately help in identification of the functional role of CaM-Gag interactions in the HIV replication cycle. PMID: 21799007
- Ca(2+) influx regulates assembly of a fully active CaN-calmodulin complex selectively on the tail of dynIxb and the complex is recruited to sites of activity-dependent bulk endocytosis in nerve terminals PMID: 21730063
- These results indicate that the aberrant formation of the activation link between CaMBD [(calmodulin)-binding domain] and CaMLD (CaM-like domain) of RyR is a key step in the development of hypertrophy in cultured cardiomyocytes. PMID: 21649588
- Calcium/calmodulin interferes with the association of AKAP150 with TRPV1. PMID: 21569553
- Data indicate that, in lactational rats, hippocampal neurogranin, CaMKII, calmodulin and calcineurin are involved in the brain impairment by developmental iodine deficiency and hypothyroidism. PMID: 20654708
- The BD-N and BD-C2 binding domains are sufficient for CaM binding to the native channel and BD-C1 is unable to bind CaM independently. PMID: 20523736
- translocation of CaM and CaMKII from the cytoplasm to the nucleus serves as messengers to transmit the pathogenic signal elicited in the surface membrane and in the RyR2 to the nuclear transcriptional sites to activate hypertrophy. PMID: 20433809
- CaM acts as a mediator in the Ca2+-dependent modulation of KCNQ channels. PMID: 12032157
- Calmodulin activity is critical for activation of volume-regulated anion channels in rat cerebral astrocytes. PMID: 15095369
- the majority of CaM nuclear entry occurs by facilitated mechanisms in all cell types examined, in part by a Ca2+-independent and in part by a Ca2+-dependent translocation mechanism PMID: 15522886
- the Ral-CaM complex defines a multifaceted regulatory mechanism for PLC-delta1 activation PMID: 15817490
- binding of 14-3-3, calmodulin and calcium channel beta-subunits to Kir/Gem is mutually exclusive PMID: 15860732
- These results explain how Calmodulin and iNOS coordinately function to form a stable complex that functions within the first 30 min following bacterial infection to upregulate the innate immune system involving macrophage activation. PMID: 16893173
- Ca2+-dependent CaM cascade might contribute to NMDA induced activation of PI-3K/Akt pathway. PMID: 17492691
- This study provides the first evidence that CaM and PKCdelta organize actin dynamics in the early endosomal compartment, thereby regulating the intracellular trafficking of EGFR. PMID: 17959830
- analysis of conformational changes of calmodulin upon Ca2+ binding PMID: 18178620
- The solution structures of complexes between calcium-saturated calmodulin (Ca (2+)/CaM) and a CaM-binding domain of the HIV-1 matrix protein p17 have been determined by small-angle X-ray scattering. PMID: 18553937
- Diabetes-induced acceleration of I(to) current inactivation is due to a reduced effect of CaMKII on I(to) channels as a result of a diabetes-induced reduction in calmodulin protein expression. PMID: 19088444
- In the intact SK channel complex, the N-lobe of calmodulin provides ligand-binding sites for channel gating, and that its ligand-binding properties are comparable to those of the N-lobe in isolated calmodulin. PMID: 19144926
- The oxidation-induced loss of secondary structure, as measured by circular dichroism, correlated with the rate of degradation for wild-type and mutant calmodulin containing Leu substitutions in the C-terminus. PMID: 19231837
- CaM bound to KCNQ2 acts as a Ca2+ sensor, conferring Ca2+ dependence to the trafficking of the channel to the plasma membrane and fully explaining the requirement of CaM binding for KCNQ2 function. PMID: 19494108
显示更多
收起更多
-
亚细胞定位:Cytoplasm, cytoskeleton, spindle. Cytoplasm, cytoskeleton, spindle pole. Cytoplasm, cytoskeleton, microtubule organizing center, centrosome.
-
蛋白家族:Calmodulin family
-
数据库链接:
KEGG: rno:24242
UniGene: Rn.129719
Most popular with customers
-
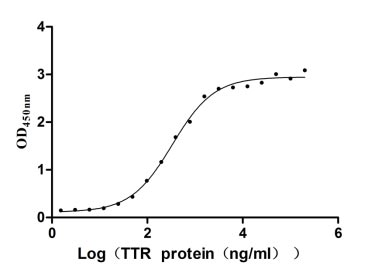
Recombinant Human Transthyretin (TTR) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Pro-neuregulin-1, membrane-bound isoform (NRG1), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
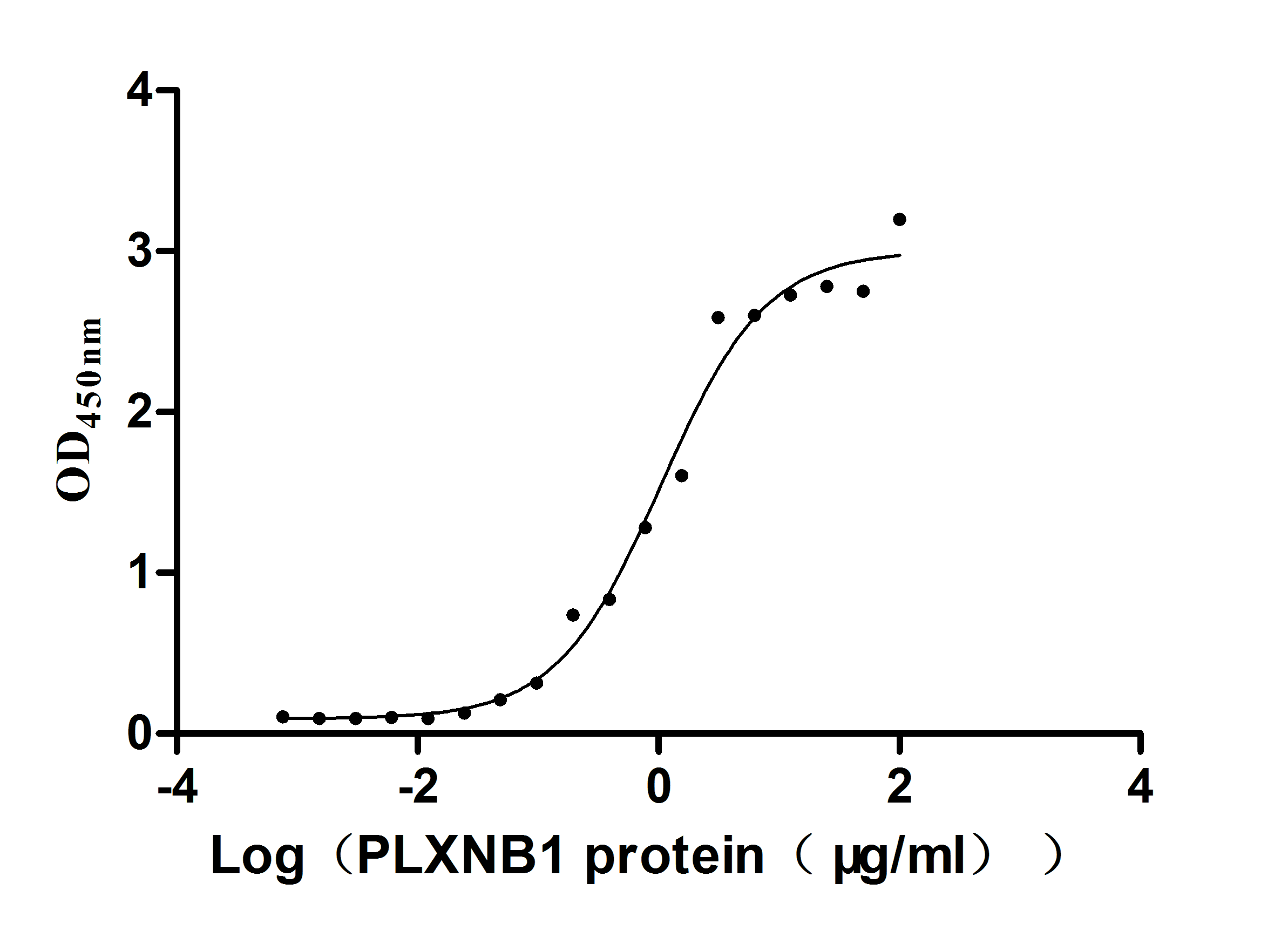
Recombinant Human Plexin-B1 (PLXNB1), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Poliovirus receptor (PVR) (I340M), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
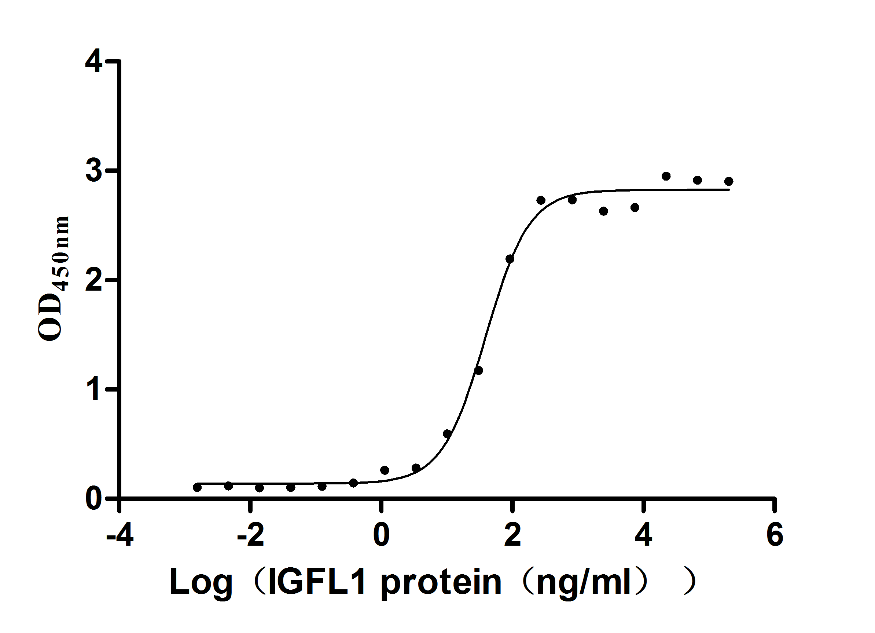
Recombinant Human IGF-like family receptor 1 (IGFLR1), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
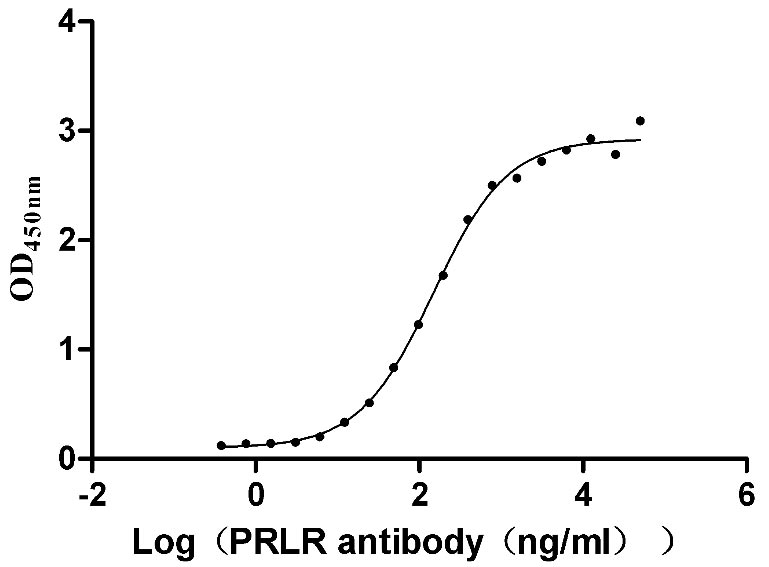
Recombinant Human Prolactin receptor (PRLR), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
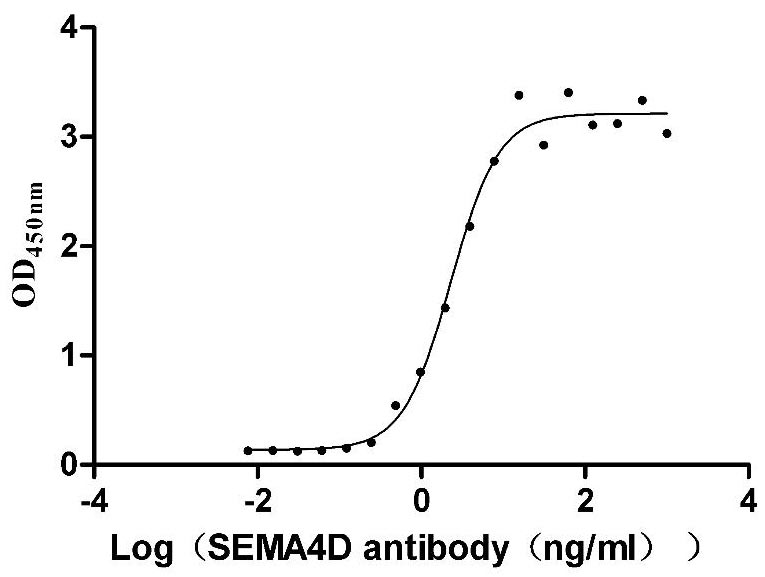
Recombinant Macaca mulatta Semaphorin-4D isoform 1 (SEMA4D), partial (Active)
Express system: Mammalian cell
Species: Macaca mulatta (Rhesus macaque)
-
Recombinant Mouse Transthyretin (Ttr) (Active)
Express system: Mammalian cell
Species: Mus musculus (Mouse)




-AC1.jpg)
-AC1.jpg)