Recombinant Human Protein syndesmos (NUDT16L1)
-
中文名称:人NUDT16L1重组蛋白
-
货号:CSB-YP863105HU
-
规格:
-
来源:Yeast
-
其他:
-
中文名称:人NUDT16L1重组蛋白
-
货号:CSB-EP863105HU
-
规格:
-
来源:E.coli
-
其他:
-
中文名称:人NUDT16L1重组蛋白
-
货号:CSB-EP863105HU-B
-
规格:
-
来源:E.coli
-
共轭:Avi-tag Biotinylated
E. coli biotin ligase (BirA) is highly specific in covalently attaching biotin to the 15 amino acid AviTag peptide. This recombinant protein was biotinylated in vivo by AviTag-BirA technology, which method is BriA catalyzes amide linkage between the biotin and the specific lysine of the AviTag.
-
其他:
-
中文名称:人NUDT16L1重组蛋白
-
货号:CSB-BP863105HU
-
规格:
-
来源:Baculovirus
-
其他:
-
中文名称:人NUDT16L1重组蛋白
-
货号:CSB-MP863105HU
-
规格:
-
来源:Mammalian cell
-
其他:
产品详情
-
纯度:>85% (SDS-PAGE)
-
基因名:NUDT16L1
-
Uniprot No.:
-
别名:NUDT16L1; SDOS; TIRR; Tudor-interacting repair regulator protein; NUDT16-like protein 1; Protein syndesmos
-
种属:Homo sapiens (Human)
-
蛋白长度:full length protein
-
表达区域:1-211
-
氨基酸序列MSTAAVPELK QISRVEAMRL GPGWSHSCHA MLYAANPGQL FGRIPMRFSV LMQMRFDGLL GFPGGFVDRR FWSLEDGLNR VLGLGLGCLR LTEADYLSSH LTEGPHRVVA HLYARQLTLE QLHAVEISAV HSRDHGLEVL GLVRVPLYTQ KDRVGGFPNF LSNAFVSTAK CQLLFALKVL NMMPEEKLVE ALAAATEKQK KALEKLLPAS S
-
蛋白标签:Tag type will be determined during the manufacturing process.
The tag type will be determined during production process. If you have specified tag type, please tell us and we will develop the specified tag preferentially. -
产品提供形式:Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
复溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
储存条件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保质期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
货期:Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事项:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet :Please contact us to get it.
靶点详情
-
功能:Key regulator of TP53BP1 required to stabilize TP53BP1 and regulate its recruitment to chromatin. In absence of DNA damage, interacts with the tandem Tudor-like domain of TP53BP1, masking the region that binds histone H4 dimethylated at 'Lys-20' (H4K20me2), thereby preventing TP53BP1 recruitment to chromatin and maintaining TP53BP1 localization to the nucleus. Following DNA damage, ATM-induced phosphorylation of TP53BP1 and subsequent recruitment of RIF1 leads to dissociate NUDT16L1/TIRR from TP53BP1, unmasking the tandem Tudor-like domain and allowing recruitment of TP53BP1 to DNA double strand breaks (DSBs). Binds U8 snoRNA.
-
基因功能参考文献:
- This study elucidates the mechanism by which TIRR recognizes 53BP1 Tudor and functions as a cellular inhibitor of the histone methyl-lysine readers. PMID: 29844495
- Data indicate the molecular mechanism underlying Tudor interacting repair regulator (TIRR)-mediated suppression of tumor protein p53 binding protein 1 (53BP1)-dependent DNA damage repair. PMID: 30002377
- findings identify TIRR as a new factor that influences double-strand break repair using a unique mechanism of masking the histone methyl-lysine binding function of 53BP1 PMID: 28241136
- TIRR is a novel 53BP1-interacting protein that participates in the DNA damage response PMID: 28213517
-
亚细胞定位:Nucleus.
-
蛋白家族:Nudix hydrolase family, TIRR subfamily
-
数据库链接:
HGNC: 28154
OMIM: 617338
KEGG: hsa:84309
STRING: 9606.ENSP00000306670
UniGene: Hs.513315
Most popular with customers
-
Recombinant Human T-cell surface protein tactile (CD96), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
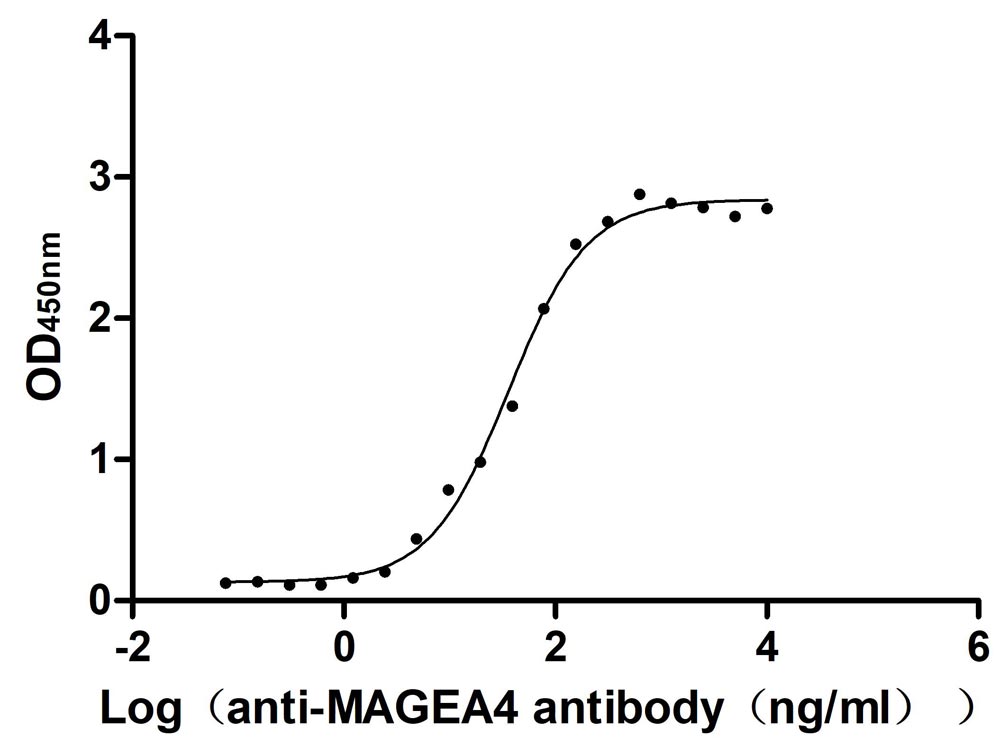
Recombinant Human Melanoma-associated antigen 4 (MAGEA4) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
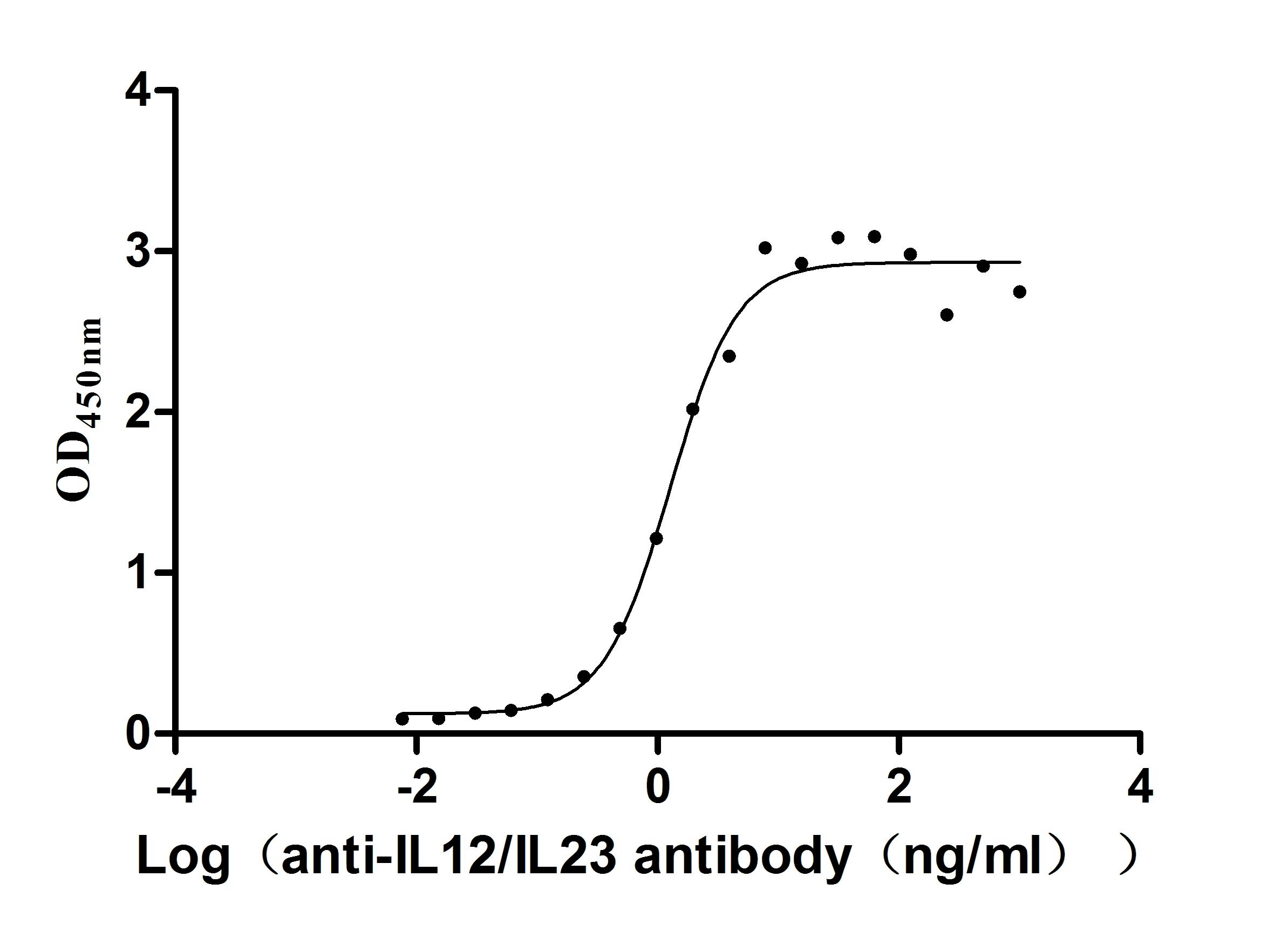
Recombinant Human IL12B&IL12A Heterodimer Protein (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
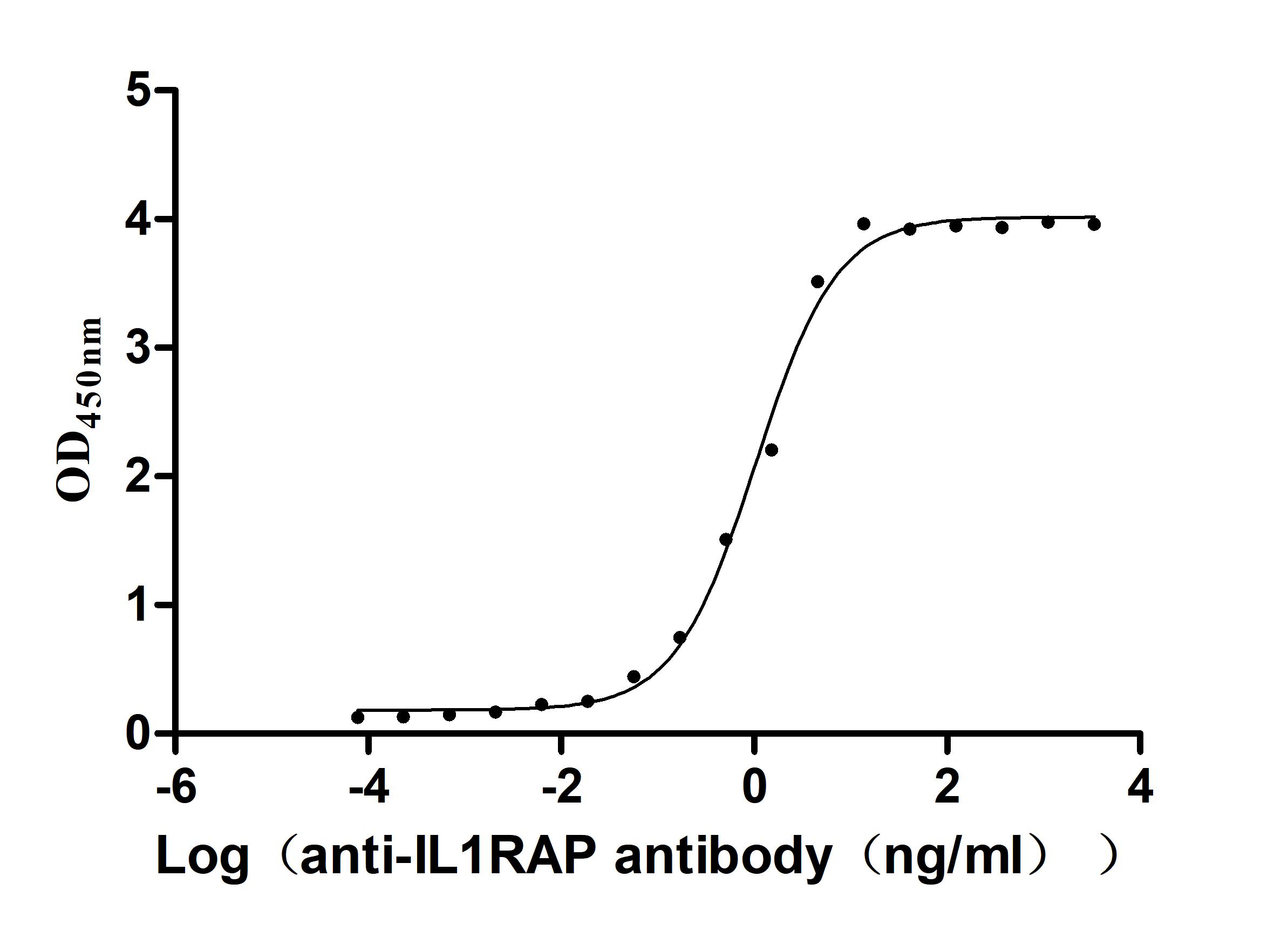
Recombinant Macaca fascicularis Interleukin 1 receptor accessory protein(IL1RAP), partial (Active)
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)
-
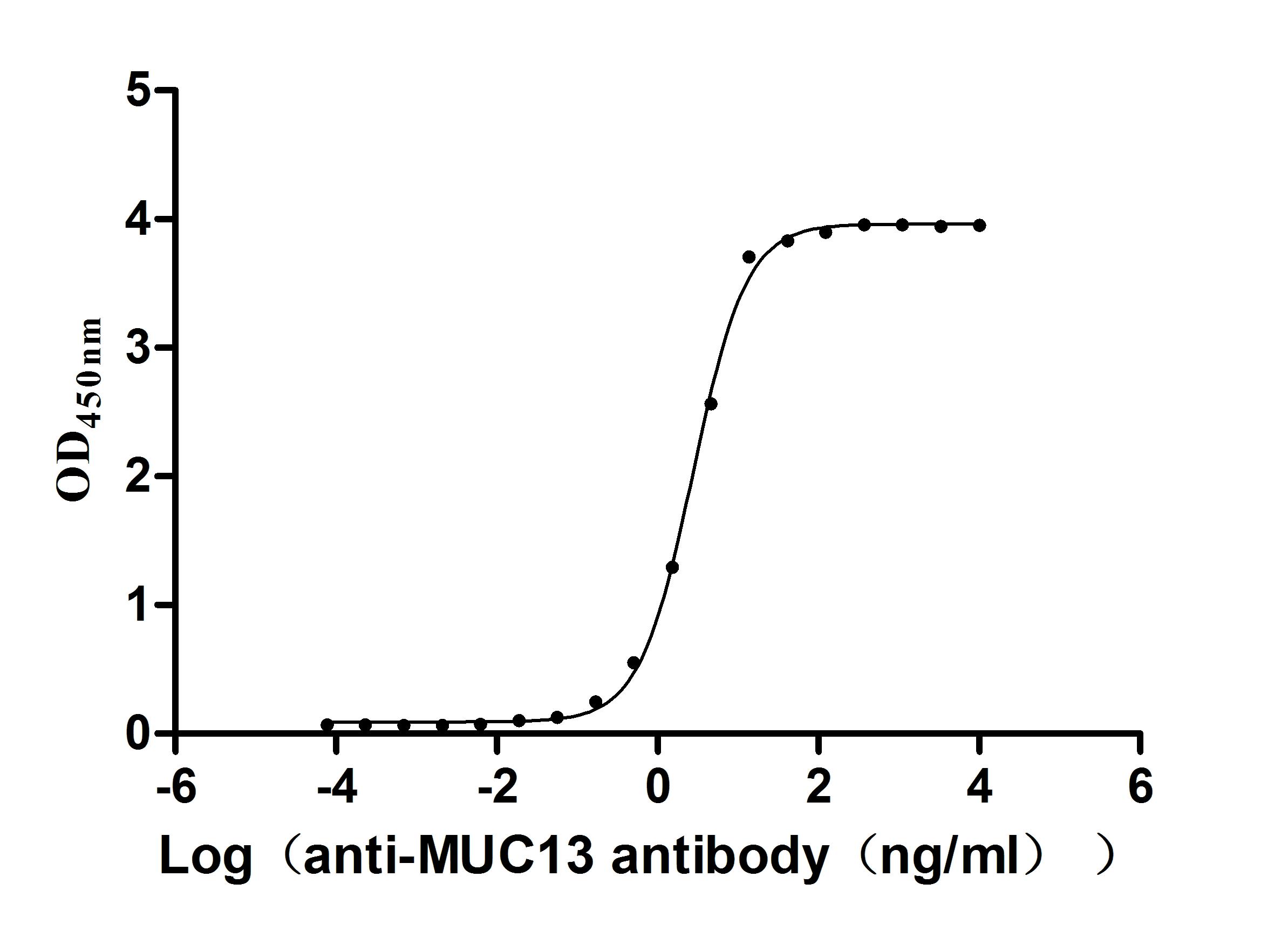
Recombinant Human Mucin-13(MUC13),partial (Active)
Express system: yeast
Species: Homo sapiens (Human)


-AC1.jpg)