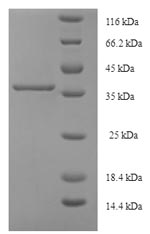
Recombinant Human Cytoglobin (CYGB)
-
中文名称:Recombinant Human Cytoglobin(CYGB)
-
货号:CSB-EP006376HU
-
规格:¥1344
-
图片:
-
其他:
产品详情
-
纯度:Greater than 90% as determined by SDS-PAGE.
-
基因名:CYGB
-
Uniprot No.:
-
别名:Cygb; CYGB_HUMAN; Cytoglobin; Hgb; Histoglobin; STAP; Stellate cell activation associated protein ; Stellate cell activation-associated protein
-
种属:Homo sapiens (Human)
-
蛋白长度:Full Length
-
来源:E.coli
-
分子量:37.4kDa
-
表达区域:1-190aa
-
氨基酸序列MEKVPGEMEIERRERSEELSEAERKAVQAMWARLYANCEDVGVAILVRFFVNFPSAKQYFSQFKHMEDPLEMERSPQLRKHACRVMGALNTVVENLHDPDKVSSVLALVGKAHALKHKVEPVYFKILSGVILEVVAEEFASDFPPETQRAWAKLRGLIYSHVTAAYKEVGWVQQVPNATTPPATLPSSGP
Note: The complete sequence including tag sequence, target protein sequence and linker sequence could be provided upon request. -
蛋白标签:N-terminal 6xHis-SUMO-tagged
-
产品提供形式:Liquid or Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
缓冲液:Tris-based buffer,50% glycerol
-
储存条件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保质期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
货期:Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事项:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet & COA:Please contact us to get it.
相关产品
靶点详情
-
功能:May have a protective function during conditions of oxidative stress. May be involved in intracellular oxygen storage or transfer.
-
基因功能参考文献:
- The results obtained in this study (1) show that plasma-produced reactive oxygen and nitrogen species can extensively oxidize proteins and (2) that the oxidation status of two redox-active cysteines lead to different conformations of CYGB. PMID: 30081385
- Endothelial cells facilitate the ability of smooth muscle cells to metabolize nitric oxide through upregulation of cytoglobin. PMID: 29969687
- FGF2 initiates CYGB transcription via the JNK pathway. PMID: 28916723
- the study reveals a novel mechanism for the regulated expression of Cygb and also assigns a new role to Cygb in cell cycle control. PMID: 28948618
- Data suggest that cytochrome b5 (CYB5) and cytochrome b5 reductase 3 (CYB5R3) can reduce human cytoglobin (CYGB) and zebrafish cytoglobins at rates up to 250-fold higher than those reported for the known physiological substrates, hemoglobin and myoglobin; the three proteins (CYB5+CYB5R3+CYGB) appear to constitute a metabolon involved in generation of nitric oxide. PMID: 28671819
- DeltaNp63-CYGB axis is also present in lung and breast cancer cell lines, indicating that CYGB-mediated ROS-scavenging activity may also have a role in epithelial tumours PMID: 26096935
- Propose a bipartite lipid binding model that rationalizes the modes of interactions of cytoglobin with phospholipids, the effects on structural re-arrangements and the peroxidase activity of the hemoprotein. PMID: 26928591
- This review provides an overview of the proposed role of cytoglobin and explores its potential functional role as a biomarker for cancer and other diseases PMID: 26339645
- Cygb, expressed in hepatic stellate cells during liver fibrosis, plays role in cancer development with nonalcoholic steatohepatitis. PMID: 25665792
- Cygb stabilizes p53 by inhibiting its ubiquitination and elicit cell cycle arrest in DNA damaged cells. PMID: 25269893
- The cysteine redox state of the monomer controls histidine dissociation rate constants and hence extrinsic ligand binding in human cytoglobin. PMID: 25601563
- The monomeric cytoglobin protein with an internal disulfide bond between the two cysteine residues Cys38 and Cys83, interacts with lipids to induce a change in haem co-ordination. PMID: 25327890
- Protein multimerization may be a mechanism that triggers physiological functions of human cytoglobin. PMID: 24632414
- Our data provides evidence that cytoglobin regulates the ovarian cancer cell proliferation and invasion. PMID: 24737588
- This review outlines the current understanding of Cygb's involvement in tumor hypoxia and discusses its role in tumorigenesis. PMID: 24816917
- Molecular dynamics studies of four cytoglobinCO models indicated that the distal E7 residue was a crucial influence on the dynamics of cytoglobinCO in terms of loop fluctuations, cavity rearrangement, and slight heme motion. PMID: 24037220
- Reduction of the internal disulfide bond between Cys 38 and 83 switches the ligand migration pathway in cytoglobin. PMID: 24008134
- Cytoglobin is expressed in hepatic stellate cells, but not in myofibroblasts, in normal and fibrotic human liver, so it thus a useful marker to distinguish these cells. PMID: 24296877
- Results show that CYGB revealed Tumor Suppressor Gene properties in normoxia but promoted tumourigenic potential of the cells exposed to stress, suggesting a bimodal function in lung tumourigenesis. PMID: 23591990
- Reduction of Cygb by cellular reductants enables Cygb to efficiently regulate nitric oxide metabolism in the vascular wall in an oxygen-dependent manner. PMID: 23710929
- Report cytoglobin expression in human brain. PMID: 23160832
- A substantial change in both protein dynamics and inner cavities is observed upon transition from the CO-liganded to the pentacoordinated and bis-histidyl hexacoordinated species, which could be exploited as a signalling state. PMID: 23308092
- Cygb-mediated nitrite reduction can play an important role in NO generation and soluble guanylyl cyclase activation under hypoxic conditions PMID: 22896706
- This suggests that Cytoglobin is likely not important for global neuronal protection following ischemia and the role of Cytoglobin in relation to endogenous neuroprotection remains unresolved. PMID: 22750003
- Coexistence of Cygb with efficient reductants in tissues allows Cygb to function as an oxygen-dependent regulator of nitric oxide (NO) decay. A related kinetic model predicts the NO consumption rate. PMID: 22577939
- normal physiological concentrations of cytoglobin do not offer cytoprotection from reactive oxygen species PMID: 22359545
- Cytoglobin, a protein that can be induced in response to oxidative stress, is elevated in most atrophic foci in adenocarcinoma of the prostate, suggesting hypoxic, and/or oxidative damage. PMID: 22025306
- knockdown of cytoglobin expression can sensitize human glioma cells to oxidative stress PMID: 21631290
- Binding of ferric cytoglobin to lipids and their subsequent transformation may be integral to the physiological function of cytoglobin, generating cell signalling lipid molecules under an oxidative environment. PMID: 21171964
- Cygb has a nitric-oxide dioxygenase function and ascorbate and cytochrome b(5) have roles as reductants PMID: 20511233
- Cytoglobin displays biphasic kinetics after the photolysis of CO, as a result of competition with an internal protein ligand, the E7 distal histidine. PMID: 20553503
- A ubiquitously expressed human hexacoordinate hemoglobin PMID: 11893755
- vertebrate myoglobins are in fact a specialized intracellular globin that evolved in adaptation to the special needs of muscle cells PMID: 11919282
- cloned, deduced amino acid sequence and expressed in diseased liver tissue where stellate cells were present PMID: 12359339
- characterization of the heme environmental structure of this protein, a fourth globin in humans PMID: 12718557
- differential expression of cytoglobin argues against a general respiratory function of this molecule, but rather indicates a connective tissue-specific function PMID: 14660570
- hereditary neuralgic amyotrophy is not caused by point mutations of cytoglobin PMID: 15052627
- Results describe the crystal structure of cytoglobin, which displays heme hexa-coordination. PMID: 15095869
- reporting of X-ray crystallographic structure PMID: 15165856
- Cytoglobin is a novel candidate tumour suppressor gene highly methylated in upper aero-digestive tract squamous cancer PMID: 16449996
- We now show that cytoglobin gene expression in oesophageal biopsies from tylotic patients is dramatically reduced by approximately 70% compared with normal oesophagus. Furthermore, both alleles are equally repressed PMID: 16510494
- Results provide the first evidence to suggest the implication of CYGB in the pathogenesis of non-small cell lung cancer. PMID: 16698880
- The structure of a new crystal form of cytoglobin reveals a new dimerization arrangement of cytoglobin. PMID: 16699195
- Pomoter elements of human CYGB gene are located between -1113 to -10 relative to the translation start site. PMID: 16797742
- hypoxia responsive elements (HREs) at positions -141, -144 and -448 were essential for activation of CYGB expression under hypoxic conditions. The binding of hypoxia inducible factor protein to the HREs was confirmed. PMID: 17936249
- A role for cytoglobin in cytoprotection of neuronal cells from oxidative-related damage. PMID: 18353768
- Data constitute the first direct functional evidence for CYGB, the newest member of the globin family, as a tumor suppressor gene. PMID: 18794132
- cytoglobin contributes to cell-mediated NO dioxygenation and represents an important NO sink in the vascular wall. PMID: 19147491
- CYGB gene is regulated by both promoter methylation and tumour hypoxia in HNSCC and that increased expression of this gene correlates with clincopathological measures of a tumour's biological aggression. PMID: 19568272
显示更多
收起更多
-
亚细胞定位:Cytoplasm.
-
蛋白家族:Globin family
-
组织特异性:Ubiquitously expressed. Highest expression in heart, stomach, bladder and small intestine.
-
数据库链接:
HGNC: 16505
OMIM: 608759
KEGG: hsa:114757
STRING: 9606.ENSP00000293230
UniGene: Hs.95120
Most popular with customers
-
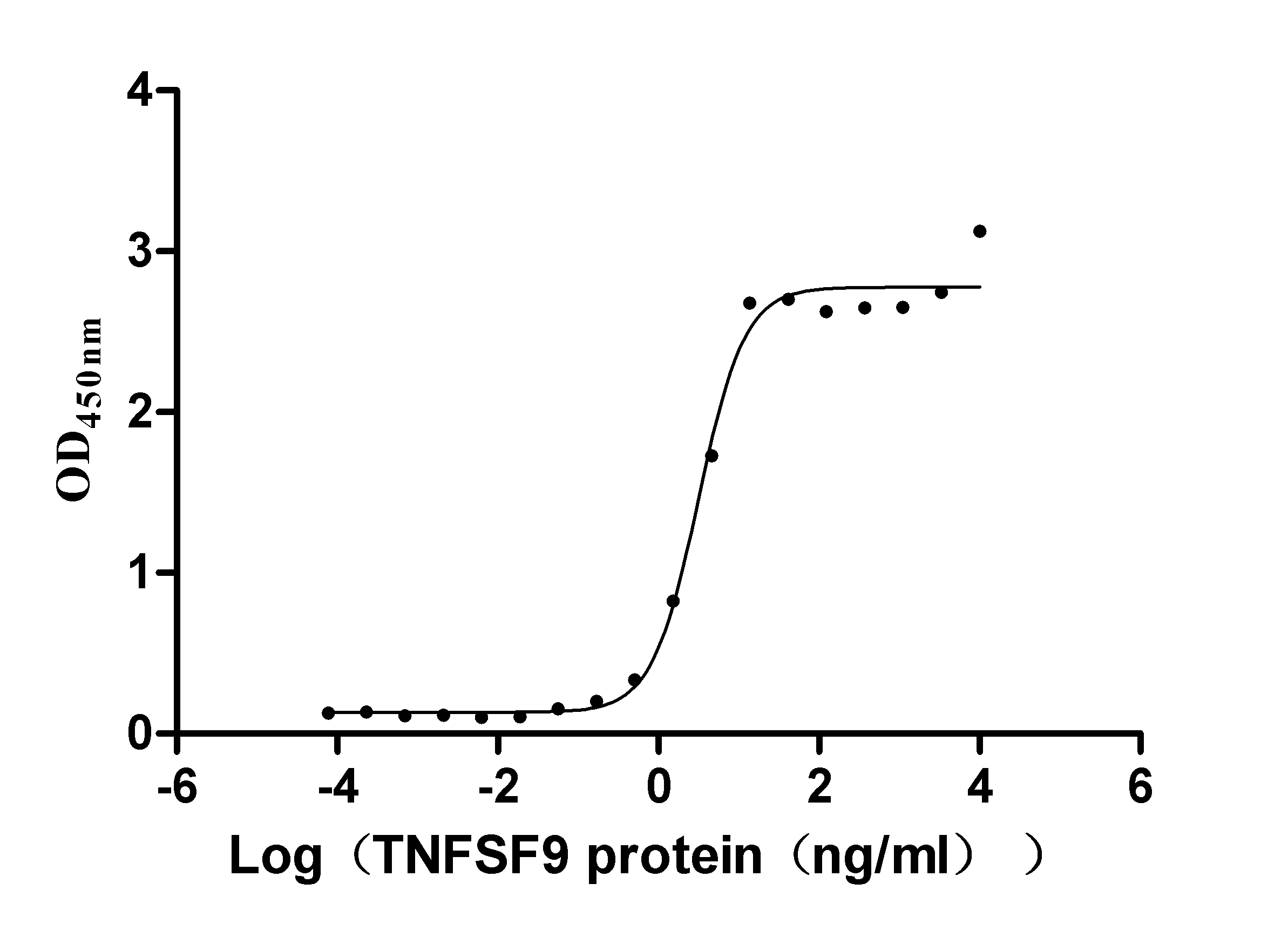
Recombinant Human Tumor necrosis factor ligand superfamily member 9 (TNFSF9), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
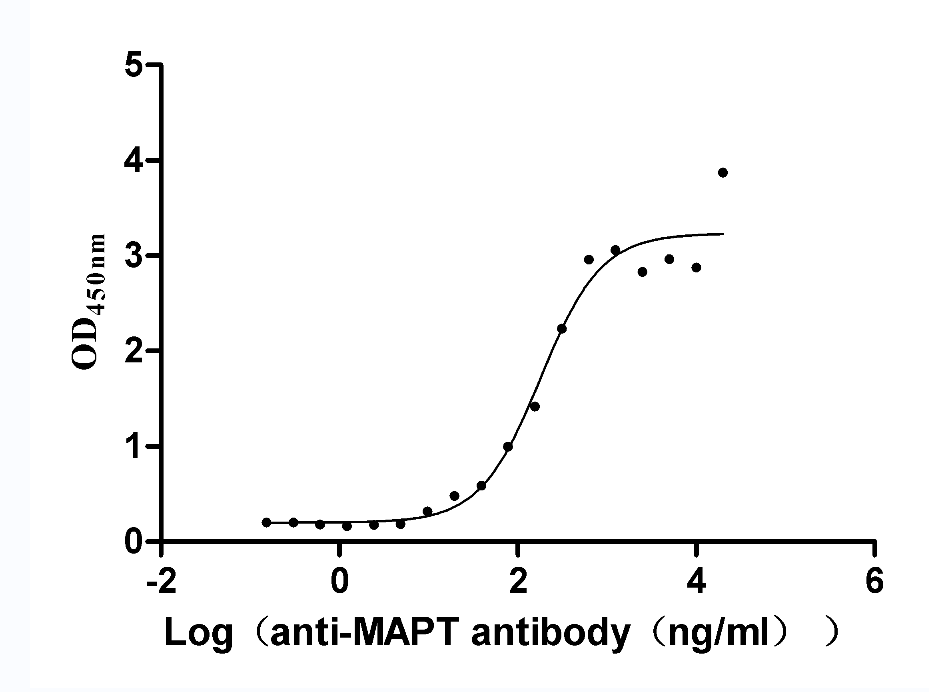
Recombinant Rat Microtubule-associated protein tau (Mapt) (Active)
Express system: Mammalian cell
Species: Rattus norvegicus (Rat)
-
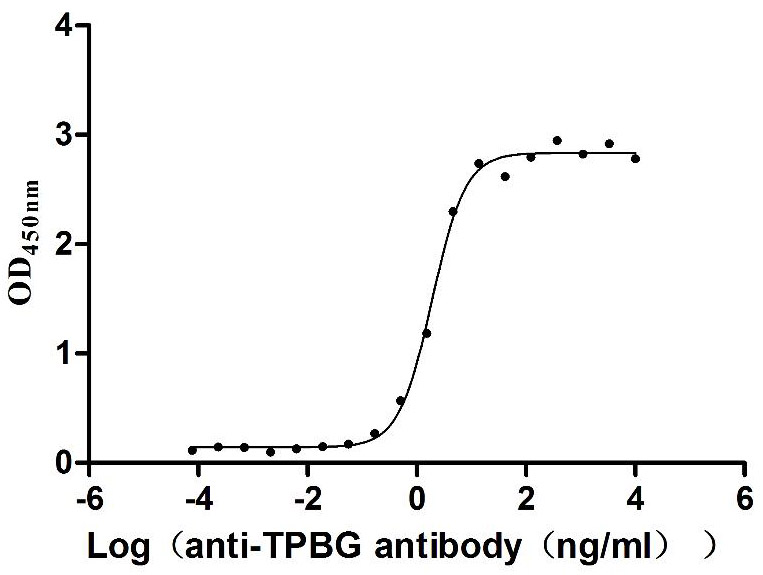
Recombinant Macaca fascicularis Trophoblast glycoprotein (TPBG), partial (Active)
Express system: Mammalian cell
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)
-
Recombinant Human Myosin regulatory light polypeptide 9 (MYL9) (Active)
Express system: Yeast
Species: Homo sapiens (Human)
-
Recombinant Human Interleukin-2 (IL2) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Macaca fascicularis Zinc transporter ZIP6 isoform X1(SLC39A6),partial (Active)
Express system: Baculovirus
Species: Macaca fascicularis (Crab-eating macaque) (Cynomolgus monkey)