Recombinant Epstein-Barr virus Latent membrane protein 1 (LMP1), partial
-
货号:CSB-YP355987EFA1
-
规格:
-
来源:Yeast
-
其他:
-
货号:CSB-EP355987EFA1
-
规格:
-
来源:E.coli
-
其他:
-
货号:CSB-EP355987EFA1-B
-
规格:
-
来源:E.coli
-
共轭:Avi-tag Biotinylated
E. coli biotin ligase (BirA) is highly specific in covalently attaching biotin to the 15 amino acid AviTag peptide. This recombinant protein was biotinylated in vivo by AviTag-BirA technology, which method is BriA catalyzes amide linkage between the biotin and the specific lysine of the AviTag.
-
其他:
-
货号:CSB-BP355987EFA1
-
规格:
-
来源:Baculovirus
-
其他:
-
货号:CSB-MP355987EFA1
-
规格:
-
来源:Mammalian cell
-
其他:
产品详情
-
纯度:>85% (SDS-PAGE)
-
基因名:LMP1
-
Uniprot No.:
-
别名:LMP1; BNLF1; Latent membrane protein 1; LMP-1; Protein p63
-
种属:Epstein-Barr virus (strain B95-8) (HHV-4) (Human herpesvirus 4)
-
蛋白长度:Partial
-
蛋白标签:Tag type will be determined during the manufacturing process.
The tag type will be determined during production process. If you have specified tag type, please tell us and we will develop the specified tag preferentially. -
产品提供形式:Lyophilized powder
Note: We will preferentially ship the format that we have in stock, however, if you have any special requirement for the format, please remark your requirement when placing the order, we will prepare according to your demand. -
复溶:We recommend that this vial be briefly centrifuged prior to opening to bring the contents to the bottom. Please reconstitute protein in deionized sterile water to a concentration of 0.1-1.0 mg/mL.We recommend to add 5-50% of glycerol (final concentration) and aliquot for long-term storage at -20℃/-80℃. Our default final concentration of glycerol is 50%. Customers could use it as reference.
-
储存条件:Store at -20°C/-80°C upon receipt, aliquoting is necessary for mutiple use. Avoid repeated freeze-thaw cycles.
-
保质期:The shelf life is related to many factors, storage state, buffer ingredients, storage temperature and the stability of the protein itself.
Generally, the shelf life of liquid form is 6 months at -20°C/-80°C. The shelf life of lyophilized form is 12 months at -20°C/-80°C. -
货期:Delivery time may differ from different purchasing way or location, please kindly consult your local distributors for specific delivery time.Note: All of our proteins are default shipped with normal blue ice packs, if you request to ship with dry ice, please communicate with us in advance and extra fees will be charged.
-
注意事项:Repeated freezing and thawing is not recommended. Store working aliquots at 4°C for up to one week.
-
Datasheet :Please contact us to get it.
相关产品
靶点详情
-
功能:Acts as a CD40 functional homolog to prevent apoptosis of infected B-lymphocytes and drive their proliferation. Functions as a constitutively active tumor necrosis factor receptor that induces the activation of several signaling pathways, including those of the NF-kappa-B family. LMP1 signaling leads to up-regulation of antiapoptotic proteins and provide growth signals in latently infected cells. Interacts with host UBE2I and subsequently affects the sumoylation state of several cellular proteins. For example, induces the sumoylation of host IRF7 thereby limiting its transcriptional activity and modulating the activation of innate immune responses. Inhibits also host IFN-alpha-stimulated STAT2 nuclear translocation and interferon-stimulated response element transcriptional activity by interacting with and inhibiting host TYK2.
-
基因功能参考文献:
- These findings highlight the viral role of Epstein-Barr virus-encoded miRNA-BART16 in regulating oncogenic LMP1 expression and a negative association with the proliferation of Epstein-Barr virus-associated gastric carcinoma. PMID: 30077726
- STRUCTURAL AND FUNCTIONAL CHARACT ERISTICS OF THE LMP1 ONCOGENE IN PATIENTS WITH TUMORS ASSOCIATED AND NOT ASSOCIATED WITH THE EPSTEIN-BARR VIRUS. PMID: 30380210
- LMP1 functions to constitutively activate NF-kappaB signalling during nasopharynx cancer pathogenesis. PMID: 28098136
- These results suggest that VM formation is increased by EBVLMP1 via VEGF/VEGFR1 signaling and provide additional information to clarify the role of EBVLMP1 in nasopharyngeal carcinoma (NPC)pathophysiology PMID: 29749553
- The results demonstrate that, together, the N terminus and transmembrane region 1 of LMP1 are sufficient for efficient sorting into small membrane-enclosed extracellular vesicles. PMID: 29950415
- LMP1 signaling alone is sufficient to induce a prominent cytotoxic CD4 response. PMID: 29311309
- LMP1/2A caused massive plasmablast outgrowth, organ damage, and death. RNA-sequencing analyses identified EBV oncoprotein effects on GC B-cell target genes, including up-regulation of multiple proinflammatory chemokines and master regulators of plasma cell differentiation. PMID: 28351978
- timed expression of LMP1 together with LMP2A in subsets of mouse B cells allows one to study major clinically relevant features of human EBV infection in vivo, opening the way to new therapeutic approaches. PMID: 27856754
- Many studies provide evidence that LMP1 promotes cancer cell growth during nasopharyngeal carcinoma (NPC) development and facilitates the interaction of cancer cells with surrounding stromal cells. A small population of LMP1-expressing cells in advanced NPC is proposed to orchestrate tumor maintenance and development through cancer stem cells. LMP1 activity seems to be shifting according to tumor stage. [review] PMID: 29247573
- LMP1-mediated NF-kappaB can up-regulate DNMT3b transcription, thereby leading to relatively higher methylation intensity at PTEN CpG islands, and ultimately silencing major tumor suppressor PTEN PMID: 27223069
- PD-L1 expression positively correlated LMP1 expression in NKTCL, which was probably mediated by the MAPK/NF-kappaB pathway PMID: 27737703
- The most interesting candidates are the FO5 "QPTKDSSPPLRV" and NO4 "STTSPPAVPHNN" peptides since both bind the C-terminus LMP1 as showed by molecular docking PMID: 27650372
- LMP1-mediated glycolysis regulates IL-1beta, IL-6 and GM-CSF production through the NLRP3 inflammasome, COX-2 and P-p65 signaling pathways to enhance tumor-associated myeloid-derived suppressor cell expansion. PMID: 28732079
- High expression of LMP1 is associated with Hodgkin lymphoma. PMID: 28427406
- The VSIG4 upregulation by LMP1 was regulated at the transcriptional level via the NF-kB signaling axis. PMID: 28859984
- study found that p53 stimulates the expression of Epstein-Barr Virus LMP1 and that IRF5 may be a mediator for p53-mediated stimulation PMID: 28794023
- These results proved facilitation of the Warburg effect by LMP1 through alteration of mitochondrial function in nasopharyngeal carcinoma cells. PMID: 28827059
- Findings indicate that Epstein-Barr virus(EBV)-encoded latent membrane protein 1 (LMP1), PI3K/AKT, miR-21 and PTEN constitute a positive feedback loop and have a key role in LMP1-induced cancer stem cells (CSCs) in nasopharyngeal carcinoma (NPC). PMID: 26568302
- RPS27a enhances viral LMP1-mediated proliferation and invasion, suggesting that RPS27a interacts with LMP1 and stabilizes it by suppressing proteasome-mediated ubiquitination. PMID: 28735865
- Epstein Barr virus LMP1 directly up-regulates HIF-1A transcription and post-transcription in nasopharyngeal carcinoma cells. PMID: 27567526
- EBV-LMP1 suppresses the DNA damage response through DNA-PK/AMPK signaling to promote radioresistance in nasopharyngeal carcinoma. PMID: 27255972
- Data indicate that phosphorylation of Janus kinase 3 (JAK3) and STAT3 transcription factor (STAT3) was inhibited by latent membrane protein 1 (LMP1)-IgG. PMID: 28009988
- Methylation of LMP1, 2A and 2B promoters mediates the silencing of LMP1, 2A and 2B in EBV-associated gastric carcinomas and cell lines in varying degrees, and could be reactivated by demethylation agent and thus may contribute to the therapy of EBV-associated gastric carcinomas. PMID: 27026080
- CD63 is a critical player in LMP1 exosomal trafficking and LMP1-mediated enhancement of exosome production and may play further roles in limiting downstream LMP1 signaling. PMID: 27974566
- LMP1 and LMP2A collaborate to promote early-onset lymphomas in a mouse model, but neither protein is absolutely essential. PMID: 28077657
- Here the s report that activation of mTORC1 by Epstein-Barr virus LMP1 is a key modulator for activation of NF-kappaB signaling to mediate aerobic glycolysis via Glut-1 upregulation. PMID: 28053105
- High LMP1 expression is associated with enhanced Epstein-Barr Virus infection in Nasopharyngeal Carcinoma. PMID: 27147748
- These results suggest that differentiation of epithelial cells activates a feed-forward loop in which KLF4 and BLIMP1 first activate Epstein-Barr virus LMP1 expression and then cooperate with LMP1 to activate Z and R expression. PMID: 28179525
- In summary, the s identified a novel mechanism by which human herpesvirus 4 LMP1 drives expression of host tumor-promoting genes by blocking generation of the inhibitory histone modification H3K27me3 through PARP1 activation. PMID: 27440880
- results definitively show that LMP1 promotes IRF4 tyrosine phosphorylation and markedly stimulates its transcriptional activity through recruiting Src via P85 PMID: 27819673
- EBV infection exhibits a role in gastric cancer and NPC development; however, expression of EBVassociated proteins LMP1 and BARF1 have differential functions during tumorigenesis of these two types of cancer. PMID: 27052804
- EBER1 and EBER2, through their activation of AKT in a B-cell-specific manner, are a functionally redundant backup of latent membrane protein 1-an essential oncoprotein in Epstein-Barr virus-associated malignancies, with a main role in AKT activation. PMID: 26787829
- Data show that the copy number of Epstein-Barr virus (EBV) latent genome correlates with the viral pathogenesis, which depends on the activation level of latent membrane protein 1 (LMP1) and host NF-kappa B (NF-kappaB) signal pathway. PMID: 26517512
- Epstein-Barr virus from Burkitt Lymphoma biopsies from Africa and South America share novel LMP-1 promoter and gene variations. PMID: 26593963
- Caspase-3 mainly degraded LMP1 proteins in HeLa cells, leading to decreased NF-kappaB and STAT3 activation. Caspase-3 cleaved the consensus DNTD sequences in the CTAR3 region of LMP1. PMID: 26921582
- mechanistic analysis suggested that LMP1 overexpression induced the expression of eIF4E, while eIF4E-shRNA dramatically attenuated the increase in cell proliferation, invasion, migration and the inhibition of apoptosis triggered by LMP-1 upregulation PMID: 26397141
- miR-1 was suppressed by LMP1 and its tumour-suppressive effects were mediated chiefly by repressing K-ras expression in nasopharyngeal carcinoma. PMID: 26852690
- results suggest an association between the 30-bp del-LMP1 and XhoI-loss with nasopharyngeal carcinoma susceptibility [Meta-Analysis] PMID: 25927427
- LMP1-mediated FGFR1 activation contributes to aerobic glycolysis and transformation of epithelial cells, thereby implicating FGF2/FGFR1 signalling activation in the EBV-driven pathogenesis of nasopharyngeal carcinoma. PMID: 26096068
- Data show that p22phox expression correlated with Epstein-Barr virus (EBV) and its encoded oncoprotein, latent membrane protein 1 (LMP1) expression. PMID: 26244812
- LMP1 induces repression of several Hox genes in part via stalling of RNA polymerase II PMID: 25745994
- Data suggest that, during B-cell transformation by Epstein-Barr virus, LMP1 (EBV latent membrane protein 1) induces signaling that stimulates Lys63-polyubiquitin chain attachment to TRAF1 (TNF receptor-associated factor 1) in the B-lymphocytes. PMID: 25996949
- LMP1, a primary oncoprotein encoded by EBV, alters several functional and oncogenic properties, including transformation, cell death and survival in epithelial cells in nasopharyngeal carcinoma. (Review) PMID: 26282825
- LMP1 activates host NF-kappaB, mitogen-activated protein kinase , phosphatidylinositol 3-kinase, IRF7, and STAT pathways. [review] PMID: 26428373
- These findings suggest that the two LMP1 signaling domains modulate their combined activity and that the bcl3 transcription factor is likely responsible for some of the unique effects of CTAR1 domain on cellular expression. PMID: 25873381
- In nonendemic region for EBV-associated pathology, Russia, any strain of EBV with any structure of LMP1 with concomitant effect of additional factors may become an etiologic agent for EBV-associated neoplasia. PMID: 26510598
- LMP1 expression level is strongly correlated with extranodal NK/T-cell lymphoma in Chinese patients. PMID: 26008210
- Inhibition of LMP1-induced protein sumoylation abrogates the binding of KAP1 to Epstein-Barr Virus promoters. PMID: 25948750
- LMP1 and LMP2A jointly regulate DNA repair signaling and cell death activation with no further enhancement in the growth properties of neoplastic cells. PMID: 25972552
- Together, the findings of this study suggest that the association of LMP1 with lipid rafts is mediated at least in part through interactions with the actin cytoskeleton. PMID: 25948738
显示更多
收起更多
-
亚细胞定位:Host cell membrane; Multi-pass membrane protein.
-
蛋白家族:Herpesviridae LMP-1 family
-
数据库链接:
KEGG: vg:3783750
Most popular with customers
-
Recombinant Mouse Microtubule-associated protein tau (Mapt) (Active)
Express system: Mammalian cell
Species: Mus musculus (Mouse)
-
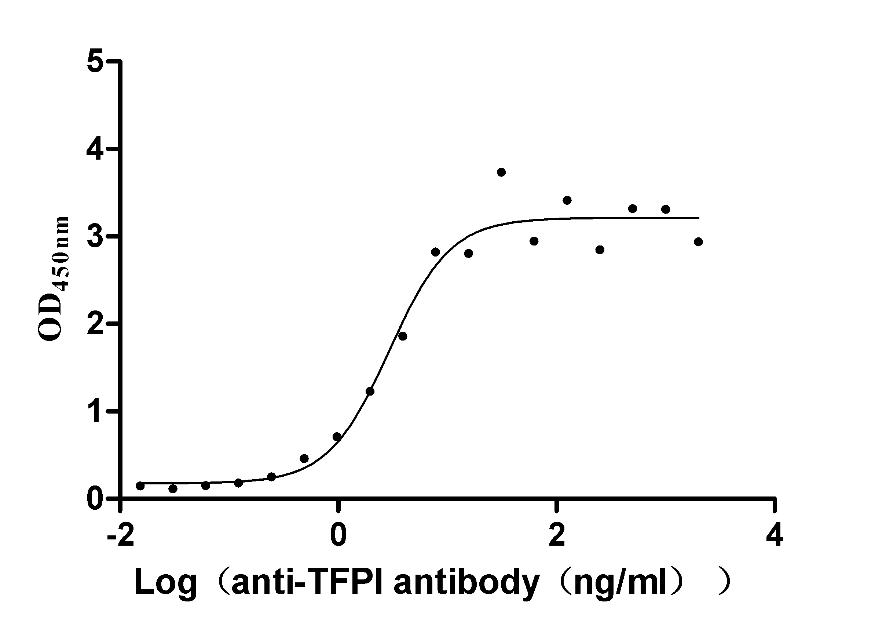
Recombinant Rabbit Tissue factor pathway inhibitor (TFPI) (Active)
Express system: Mammalian cell
Species: Oryctolagus cuniculus (Rabbit)
-
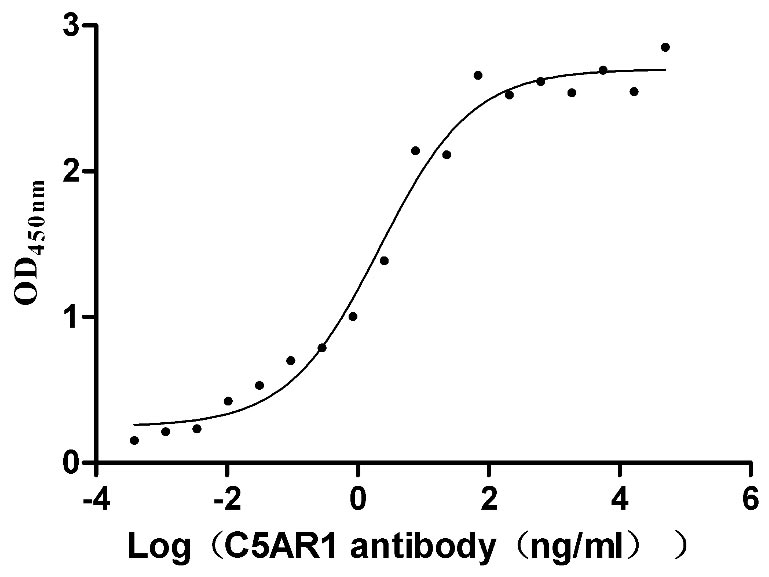
Recombinant Human C5a anaphylatoxin chemotactic receptor 1 (C5AR1)-VLPs (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
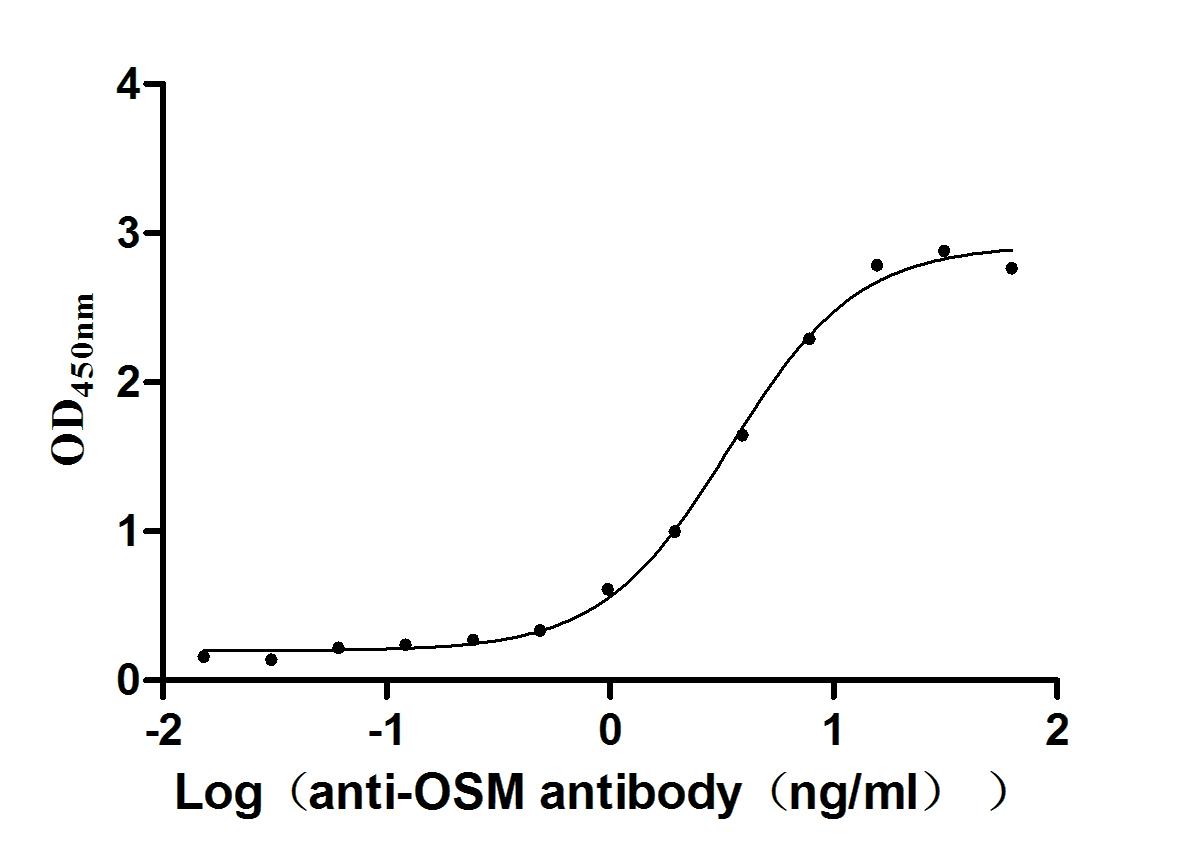
Recombinant Human Oncostatin-M (OSM), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
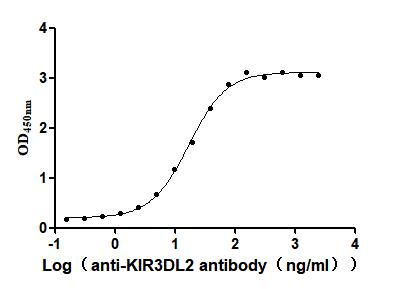
Recombinant Human Killer cell immunoglobulin-like receptor 3DL2 (KIR3DL2), partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Myosin regulatory light chain 12A (MYL12A) (Active)
Express system: E.coli
Species: Homo sapiens (Human)
-
Recombinant Human Cadherin-1(CDH1),partial (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)
-
Recombinant Human Urokinase-type plasminogen activator(PLAU) (Active)
Express system: Mammalian cell
Species: Homo sapiens (Human)